What Does the Future Hold for Acute Kidney Injury? A Deep Dive into the Evolving Pipeline
Jan 16, 2025
Table of Contents
Acute Kidney Injury is defined as a sudden advent of kidney failure or damage due to the accumulation of waste products in the bloodstream resulting in improper fluid outflow. It is profoundly evident in elderly patients who are hospitalized, especially in intensive care units. The reported incident cases range from 22% to 67% of admissions. The indication is widely associated with an array of organs that get affected, such as coronary heart disease, chronic kidney disease, diabetes, etc. It emerges as an ailment in about 5–30% of patients who undergo cardiothoracic surgery, based on the definition used for AKI. If AKI appears after major abdominal surgery, the risk of death is markedly increased (over 12-fold). Besides, it is worth noting that AKI occurs due to several clinical manifestations as well, such as sepsis, cardiac catheterization, and cardiac surgery, which are mostly hospital-acquired.
The significant unmet need that encompasses AKI is that currently, neither there are any proven therapeutic strategies to reduce post-AKI sequelae nor affirmed robust evidence to date to inform strategy for the provision of health care. Moreover, the Acute kidney injury market does not hold any approved therapy to treat it specifically, which also adds to its shortcomings.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Interstitial Cystitis: A big opportunities for emerging drugs
- Top 5 Cancers Creating Major Challenge To The Global Healthcare System
- Everything You Need to Know About Acute Kidney Injury
- Prostate Cancer Market Experiences an Influx of the Pharma Players Veering the Market Ahead
- Reblozyl under FDA review; Audentes buyout & Tecentriq’s new approval
Acute Kidney Injury Pipeline Analysis
The Acute Kidney Injury pipeline is witnessing promising developments, with various novel therapies emerging to target different underlying causes and risk factors for AKI. The increasing focus is on preventing AKI in high-risk patient groups, particularly those undergoing cardiac surgery, suffering from sepsis, or experiencing hepatic renal syndrome (HRS). These patient populations are particularly vulnerable, and current treatments do not adequately address the growing unmet need for effective prevention and treatment options. As the number of people affected by AKI continues to rise, the market for its treatments is expected to expand significantly during the forecast period from 2024 to 2034.
The emerging pipeline for AKI consists of several promising candidates aimed at providing novel therapeutic approaches for treating AKI in various high-risk settings. These include drugs designed to target AKI associated with comorbidities such as sepsis, cardiac surgery, delayed graft function, and more. There are approximately 30+ companies and 30+ pipeline drugs in the Acute Kidney Injury pipeline landscape. Some key players in the AKI pipeline include Renibus Therapeutics, Ocelot Bio, AM-Pharma, Guard Therapeutics, and others. These companies are actively developing therapies to address the critical unmet needs of AKI patients, with the potential to reduce mortality rates and improve prognosis in hospital settings.
Notable drugs currently under investigation in the pipeline include RBT-1, OCE-205, APX-115, Ilofotase alfa, and bRESCAP, among others. These candidates focus on different mechanisms, including addressing renal injury caused by systemic inflammation, oxidative stress, and ischemia-reperfusion, which are common in hospital-acquired AKI. The potential to develop more targeted, effective treatments could revolutionize AKI management, significantly improving patient outcomes.
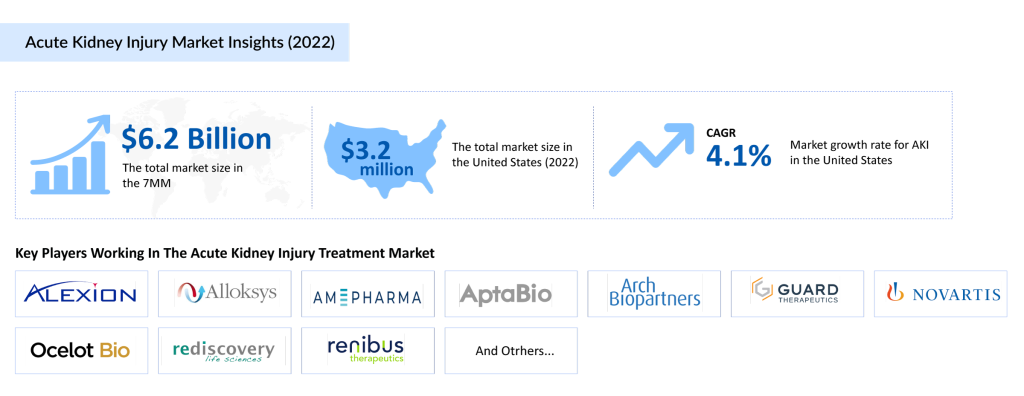
Several well-established pharmaceutical companies are also investing heavily in AKI drug development, including Alexion Pharmaceuticals, Alloksys Life Sciences, AM-Pharma, AptaBio Therapeutics, Arch Biopartners, Guard Therapeutics, Novartis Pharmaceuticals, Ocelot Bio, Rediscovery Life Sciences, Renibus Therapeutics, and River 2 Renal. These companies, with their diverse portfolios, are expected to drive innovation in the AKI space and contribute to the anticipated market growth. With a range of promising therapies in the pipeline, the future of AKI treatment looks poised for significant progress.
MOAs explored
The mechanisms of action being explored in the AKI pipeline are diverse, including:
- Angiopoietin 1 Receptor Agonists: Four drugs are currently in preclinical development targeting this receptor, which plays a role in kidney repair and regeneration.
- Alkaline Phosphatase Modulation: This MOA reduces inflammation and promotes kidney recovery.
- Cell Therapy Applications: Several companies are investigating cellular therapies to repair damaged renal tissues.
- Stem Cell Modulators: These drugs enhance kidney regeneration through stem cell mechanisms.
- Actin Modulators and Amyloid Inhibitors: These approaches target cellular processes involved in kidney injury and inflammation.
The exploration of these various MOAs indicates a comprehensive effort to tackle the underlying causes of AKI.
Key Companies and Their Lead Assets
Renibus Therapeutics’ RBT-1 is an innovative investigational drug designed to activate anti-inflammatory, antioxidant, and iron-scavenging pathways, initiating a cytoprotective preconditioning response. RBT-1 aims to reduce postoperative complications and improve recovery outcomes following cardiothoracic surgery by providing broad organ protection. With its potential to significantly enhance both short- and long-term patient recovery, RBT-1 received Breakthrough Therapy Designation (BTD) from the US FDA in July 2023. The pivotal Phase III PROTECT trial, which began in October 2023, is expected to yield top-line results by mid-2025, with a potential New Drug Application (NDA) filing planned for early 2026. This marks a significant milestone in the acute kidney injury therapeutic market, offering hope for innovative acute kidney injury therapies.
Ocelot Bio’s OCE-205 is a novel therapeutic peptide designed to address end-stage liver disease (ESLD) complications, particularly hepatorenal syndrome (HRS). By selectively targeting the vasopressin 1a (V1a) receptor and avoiding the vasopressin 2 (V2) receptor, OCE-205 aims to reduce fluid retention and improve patient outcomes. Granted Orphan Drug Designation (ODD) by the FDA in August 2022, OCE-205 is currently in Phase II clinical trials for patients with hepatorenal syndrome and acute kidney injury (HRS-AKI). Meanwhile, AptaBio Therapeutics’ APX-115, a small-molecule inhibitor targeting NADPH-oxidase (NOX) isozymes, seeks to mitigate oxidative stress-related tissue damage. Approved for Phase II clinical trials by the FDA in January 2023, APX-115 is under evaluation for contrast-induced acute kidney injury (CI-AKI), with study completion anticipated by the end of 2024. These drugs represent key additions to the acute kidney injury pipeline, addressing unmet needs in acute kidney injury therapies.
AM Pharma’s Ilofotase alfa, a recombinant alkaline phosphatase, offers a promising approach to mitigating kidney damage in sepsis-associated acute kidney injury (SA-AKI). It showed significant mortality reduction in Phase II trials, leading to the ongoing REVIVAL Phase III trial across North America, Europe, and Japan. Despite challenges with its primary endpoint, the trial continues with interim analyses focusing on additional outcomes. A Phase I pharmacokinetics study in Japan further explores its safety and efficacy. These advancements underscore the dynamic progress in the therapeutic market of acute kidney injury, with a robust pipeline paving the way for transformative treatments.
Explore the Future of AKI Treatment: Dive into Our Comprehensive Blog on the Latest Drugs in the Acute Kidney Injury Pipeline.
Conclusion
The acute kidney injury therapeutic market is on the brink of transformative advancements, driven by a robust and dynamic pipeline of innovative therapies targeting diverse mechanisms of action. From cytoprotective preconditioning and oxidative stress inhibition to vasopressin receptor modulation and alkaline phosphatase therapies, these developments reflect an unwavering commitment to addressing the significant unmet needs in acute kidney injury treatment. The ongoing clinical trials and breakthrough designations for several key drugs underline the potential for paradigm-shifting progress in acute kidney injury therapies.
As pharmaceutical leaders and biotech innovators continue to invest in acute kidney injury pipeline advancements, the future holds promise for more effective, targeted, and comprehensive solutions. These emerging therapies aim to improve survival rates and enhance long-term recovery and quality of life for patients. With increasing global attention and investment, the acute kidney injury therapeutic market is poised to redefine the standards of care, offering new hope to millions affected by this life-threatening condition.

Downloads
Article in PDF
Recent Articles
- Cholera vaccine delivered; Rice-inspired breakthroughs; International partnerships
- Alnylam’s lumasiran results; AZ’s oncology drug; Astellas Roxadustat; Evotec’s partnership with ABL
- Top 5 Cancers Creating Major Challenge To The Global Healthcare System
- Copiktra receives approval; Lilly wins approval; Eisai’s Fycompa; Astellas’ Roxadustat; Janssen’s...
- Interstitial Cystitis: A big opportunities for emerging drugs