All Blogs
Oct 02, 2024
Gastrointestinal Cancers: Exploring the Range of Digestive Tract Malignancies
With millions of cases diagnosed globally each year, gastrointestinal cancers represent a significant health challenge but also an opportunity for breakthroughs in targeted therapies and personalized medicine. Gastrointestinal cancers refer to a group of cancers that affect the digestive system, including the esoph...
Read More...
Oct 01, 2024
IntraBio’s AQNEURSA Niemann-Pick Disease Approval; FDA Approves Novel Schizophrenia Drug After 35 Years; Selpercatinib Gets FDA Nod for RET-Mutated MTC; DUPIXENT Receives First-Ever COPD Approval; Pfizer Withdraws OXBRYTA for Sickle Cell Disease from Global Market
IntraBio's AQNEURSA Receives Historic FDA Approval for Niemann-Pick Disease Type C Treatment IntraBio Inc. has received approval from the FDA for AQNEURSA (levacetylleucine), marking a significant milestone in the treatment of neurological manifestations of Niemann-Pick disease type C in both adults and pe...
Read More...
Oct 14, 2025
5 Upcoming Schizophrenia Drugs Competitors for Newly Approved BMS’ KarXT (COBENFY)
After years of little progress in the field of schizophrenia drug treatment, Bristol Myers Squibb’s USD 14 billion acquisition of Karuna Therapeutics has paid off with the approval of a groundbreaking new drug. BMS’s novel medication, KarXT—now named COBENFY—received FDA approval on 26 September 2024 for the treatm...
Read More...
Jun 10, 2025
5 Upcoming Bispecific & Trispecific Antibodies Beyond Oncology
Imagine a world where treatment options are as precise as a scalpel and as adaptable as a chameleon. Bispecific antibodies are paving the way for this future, bringing revolutionary advancements in oncology and various therapeutic fields. By harnessing the power of these dual-targeting agents, researchers are unloc...
Read More...
Sep 27, 2024
Promising Data from the First Dedicated Kidney Outcomes Trial with GLP-1 Receptor Agonist, Semaglutide, in Patients with Type 2 Diabetes and Chronic Kidney Disease
On 11 September 2024, the latest results from the FLOW trial were presented at the European Association for the Study of Diabetes (EASD) conference in Madrid, Spain. The FLOW trial, initiated in 2019, was a global, multinational, randomized controlled trial conducted across 28 countries, 387 sites, and 3,533 partic...
Read More...
Sep 27, 2024
Pooled Analysis of Finerenone Presented at EASD 2024, Poised to Strengthen its Position in the Cardio-Kidney-Metabolic Market Further
Data from an integrated pooled analysis of finerenone across three Phase III trials involving heart failure, chronic kidney disease (CKD), and Type 2 diabetes (T2D) was presented at the 2024 European Association for the Study of Diabetes (EASD) Congress. This analysis was conducted with data from the FIDELIO-DKD2 a...
Read More...
Sep 26, 2024
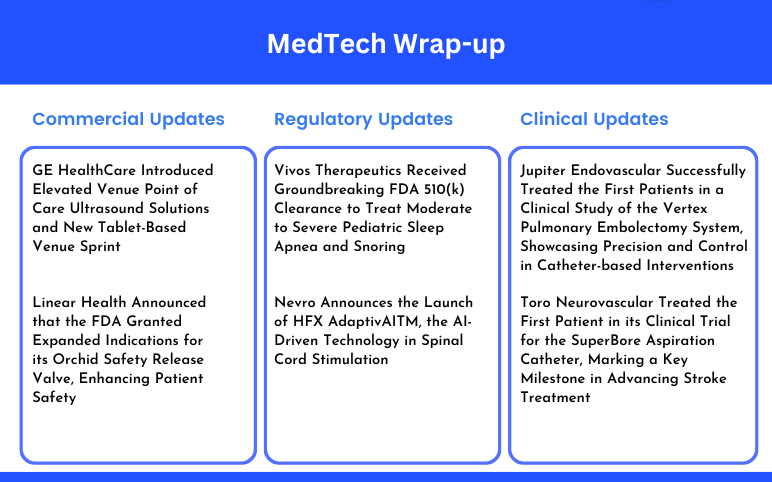
Vivos Therapeutics Receives FDA 510(k) Clearance for Pediatric Sleep Apnea; Nevro Launches AI-Driven Spinal Cord Stimulation; Jupiter Endovascular Treated First Patients in Pulmonary Embolectomy System Study; Toro Neurovascular Advances Stroke Treatment with SuperBore Aspiration Catheter; GE HealthCare Introduces Venue Ultrasound Solutions; Linear Health Expands Orchid Safety Valve Indications
Vivos Therapeutics Received Groundbreaking FDA 510(k) Clearance to Treat Moderate to Severe Pediatric Sleep Apnea and Snoring On September 18, 2024, Vivos Therapeutics, Inc., a premier medical device and technology firm focused on innovative treatments for sleep-related breathing disorders (SRBDs), including obs...
Read More...
Sep 25, 2024
Prostate Cancer Awareness Month: Early Detection Saves Lives!!
Every September, the spotlight turns to prostate cancer. Prostate cancer stands as one of the most prevalent cancers among men, affecting approximately 1 in 8 men in the United States during their lifetime. With nearly 288,000 new cases diagnosed each year, men must prioritize regular screenings and early detection...
Read More...
Sep 24, 2024
Zevra’s MIPLYFFA Niemann-Pick Disease Approval; FASENRA Approved for Eosinophilic Granulomatosis; Sanofi’s SARCLISA Gets Multiple Myeloma Approval; RYBREVANT Combo Gets FDA Nod for EGFR Lung Cancer; UCB’s BIMZELX Receives Multiple Approval from the FDA
FDA Greenlights Zevra Therapeutics’ MIPLYFFA for Niemann-Pick Disease Type C Zevra Therapeutics, Inc. has announced that the FDA has approved MIPLYFFA (arimoclomol) capsules as the first oral treatment for Niemann-Pick disease type C. Indicated for use alongside miglustat, MIPLYFFA is intended for both adult and...
Read More...
Sep 23, 2024
Roche’s TECENTRIQ HYBREZA: Setting New Standards as the First Subcutaneous Anti-PD-(L)1 Drug
Better late than never for FDA approval of the first subcutaneous PD-L1 inhibitor—Roche’s TECENTRIQ HYBREZA—after manufacturing delays disrupted the company’s initial launch plans last year. The FDA was originally set to decide on TECENTRIQ’s under-the-skin formulation in September, but Roche’s delivery technolo...
Read More...