All Blogs
Sep 23, 2024
Roche’s TECENTRIQ HYBREZA: Setting New Standards as the First Subcutaneous Anti-PD-(L)1 Drug
Better late than never for FDA approval of the first subcutaneous PD-L1 inhibitor—Roche’s TECENTRIQ HYBREZA—after manufacturing delays disrupted the company’s initial launch plans last year. The FDA was originally set to decide on TECENTRIQ’s under-the-skin formulation in September, but Roche’s delivery technolo...
Read More...
Sep 20, 2024
Artificial Intelligence (AI) in Respiratory Care: An Opportune Time for Adoption in Clinical Settings and the Drug Development Process
Artificial Intelligence (AI) is one of the most groundbreaking innovations of the 21st century, with applications spanning nearly every industry, including healthcare. In respiratory care, Artificial intelligence has the potential to enhance accuracy and efficiency while reducing costs. It can play a transformative...
Read More...
Sep 20, 2024
Post WCLC 2024 Insights: Recent Advancements in Thoracic Malignancies
NSCLC is the most common type of lung cancer accounting for approximately 85% of all lung cancers (roughly 509,000 incident cases in 2019 [7MM: the US, EU4 and the UK, and Japan]). The real-world treatment trend depicts a significant shift towards targeted and immunotherapies (from only systemic therapies in the pa...
Read More...
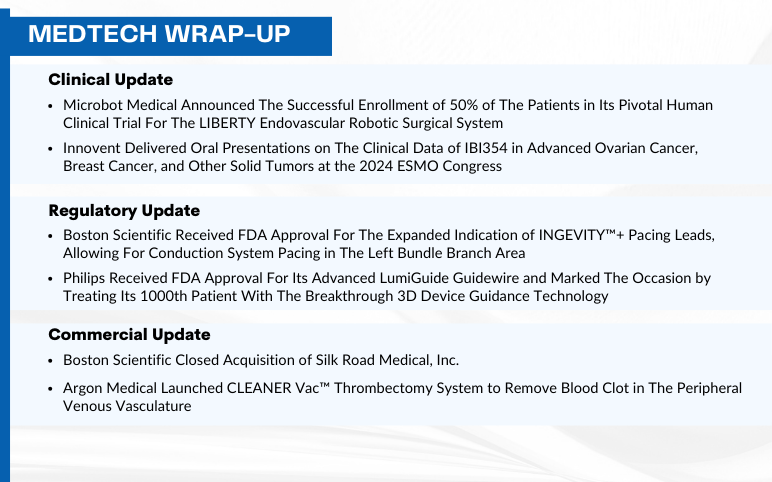
Sep 19, 2024
Boston Scientific INGEVITY™ gets Expanded Indication and acquires Silk Road Medical; FDA Approves Philips’ LumiGuide Guidewire; Microbot Completes LIBERTY Trial Enrollment; Innovent Presents Data at ESMO 2024; Argon Medical Launches CLEANER Vac™ System
Boston Scientific Received FDA Approval For The Expanded Indication of INGEVITY™+ Pacing Leads, Allowing For Conduction System Pacing in The Left Bundle Branch Area On September 17, 2024, Boston Scientific Corporation received U.S. Food and Drug Administration (FDA) approval to expand the indication for th...
Read More...
Sep 18, 2024
Tiny Organs, Big Impact: How Organoids are Shaping the Future of Medicine
Imagine a world where tiny, lab-grown organs could hold the key to unlocking the mysteries of disease, revolutionizing drug development, and personalizing medicine. Welcome to the fascinating world of organoids—miniature, self-organized clusters of cells that mimic the structure and function of real organs. In rece...
Read More...
Sep 17, 2024
Johnson & Johnson’s TREMFYA Approved for Ulcerative Colitis; Roche’s Tecentriq Hybreza Approved as Subcutaneous Anti-PD-(L)1; OCREVUS ZUNOVO Receives Twice-a-Year Multiple Sclerosis Injection Approval; Lilly’s EBGLYSS Greenlit for Moderate-to-Severe Atopic Dermatitis; DUPIXENT Approved for Adolescents with Chronic Rhinosinusitis
TREMFYA Approved for Moderately to Severely Active Ulcerative Colitis Johnson & Johnson announced that the FDA has approved TREMFYA (guselkumab) for treating adults with moderately to severely active ulcerative colitis. TREMFYA is the first fully human, dual-acting monoclonal antibody that blocks IL-23 and b...
Read More...
Sep 16, 2024
Travere’s FILSPARI Approval Sparks Rivalry in the IgA Nephropathy Space
A year after failing to meet a trial endpoint, Travere Therapeutics can now relax. On 05 September 2024, the FDA upgraded FILSPARI’s conditional approval for the kidney disease IgA nephropathy to full approval. FILSPARI’s complete approval broadens its use, permitting the small molecule blocker to help slow kidn...
Read More...
Sep 13, 2024
7 Hidradenitis Suppurativa Drugs Set to Hit the Market in the Next 5 Years
Currently, leading hidradenitis suppurativa drugs on the market include UCB Biopharma’s BIMZELX (bimekizumab), Novartis’ COSENTYX (secukinumab), and AbbVie/Eisai’s HUMIRA (adalimumab). HUMIRA dominated the market until 2023, despite its US composition-of-matter patent expiring in December 2016. AbbVie maintained a ...
Read More...
Sep 12, 2024
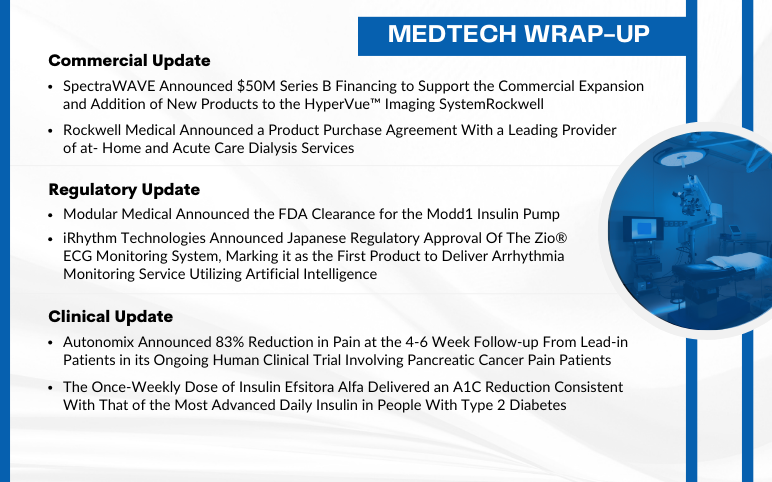
SpectraWAVE raised $50M; Rockwell Medical signed dialysis deal; Modular Medical got FDA nod for Modd1 pump; iRhythm’s Zio approved in Japan; Autonomix trial showed 83% pain reduction; Insulin Efsitora Alfa improved A1C.
Modular Medical Announced the FDA Clearance for the Modd1 Insulin Pump On September 5, 2024, Modular Medical, Inc., an insulin delivery system technology company, announced that it received the U.S. Food and Drug Administration (FDA) clearance to market and sell its MODD1 pump in the United States. With it...
Read More...
Sep 11, 2024
Top 7 Trends Shaping the Future of FemTech
The concept of FemTech has seen a surge in popularity in recent years. FemTech, short for female technology, focuses on using technology and innovation to address women’s health issues and provide solutions tailored to their unique needs. This field encompasses a broad spectrum of diagnostic tools, products, servic...
Read More...