All Blogs
Sep 11, 2024
Top 7 Trends Shaping the Future of FemTech
The concept of FemTech has seen a surge in popularity in recent years. FemTech, short for female technology, focuses on using technology and innovation to address women’s health issues and provide solutions tailored to their unique needs. This field encompasses a broad spectrum of diagnostic tools, products, servic...
Read More...
Sep 10, 2024
Biogen’s SPINRAZA Phase II/III Trial Results; Travere’s FILSPARI FDA Approval; GSK’s NUCALA Succeeds in COPD Trial; Summit’s NSCLC Win Over KEYTRUDA Raises Caution; FDA Lifts Hold on RZ358 for Congenital Hyperinsulinism
Biogen's Higher SPINRAZA Dose Shows Improved Efficacy in Phase II/III Trial A trial studying a higher dose of Biogen’s spinal muscular atrophy drug SPINRAZA (nusinersen) has met the primary endpoint in a cohort of infants with SMA. The Phase II/III DEVOTE study, which included 145 patients across various ages an...
Read More...
Sep 09, 2024
Helicobacter pylori Infections Treatment: Emerging Molecular Targets
Helicobacter pylori infection is among the most widespread chronic bacterial infections globally, affecting roughly half of the world’s population. H. pylori is classified as a Class I carcinogen by the WHO and is recognized as a qualifying pathogen under the US FDA’s GAIN Act. It is the most significant known risk...
Read More...
Sep 06, 2024
Could Hidradenitis Suppurativa Be Another Psoriasis?
Hidradenitis suppurativa is a chronic inflammatory skin disease that affects the hair follicles and apocrine sweat glands. Hidradenitis suppurativa affects approximately 1% of the population, with women being more commonly affected than men. It often begins after puberty, usually in the 20s or 30s. In 2023, the ...
Read More...
Sep 05, 2024
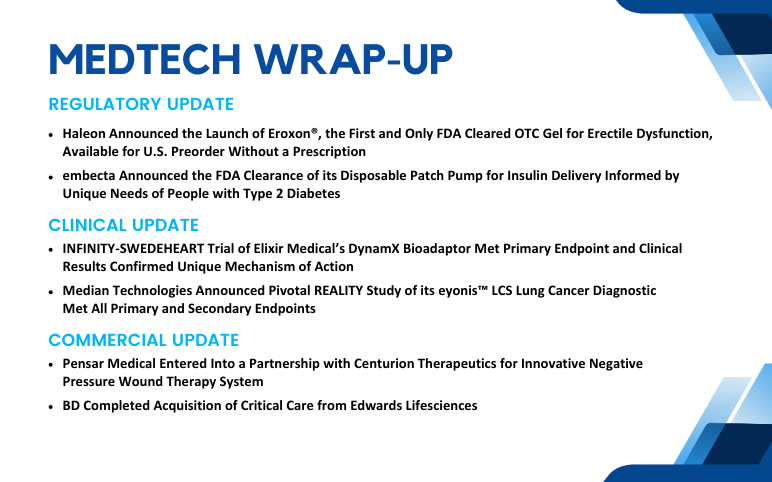
Pensar Medical’s Partnership with Centurion Therapeutics; BD’ Acquisition of Critical Care; Haleon’s Launch of Eroxon; embecta Receives FDA Clearance; INFINITY-SWEDEHEART Trial of Elixir Medical’s DynamX Bioadaptor; Median Technologies’ Pivotal REALITY Study
Pensar Medical Entered Into a Partnership with Centurion Therapeutics for Innovative Negative Pressure Wound Therapy System On Aug 29, 2024, Pensar Medical, a pioneer in negative pressure wound therapy (NPWT), announced a strategic partnership with Centurion Therapeutics to distribute the Microdoc® sNPWT (...
Read More...
Sep 04, 2024
The Protein Chip: Revolutionizing Protein Analysis and Discovery
In the rapidly evolving field of biotechnology, the quest to understand the complexity of proteins and their functions has led to the development of innovative tools and technologies. One such breakthrough is the protein chip, a powerful tool that has transformed the landscape of protein analysis. What Are Prote...
Read More...
Sep 03, 2024
Bayer Phase III NSCLC Trial; D&D Pharmatech Gets FDA Nod for GLP-1R Agonist in Multiple Scletosis; Merck Halts Two KEYTRUDA Trials; Novartis Expands LEQVIO After Phase III Success; Alnylam Reports Strong Phase III Data for Vutrisiran
Bayer Starts Phase III Trial In Non-Small Cell Lung Cancer (NSCLC) Bayer has officially enrolled the first patient in the global Phase III SOHO-02 trial, which will evaluate the efficacy and safety of BAY 2927088 as a first-line treatment for advanced non-small cell lung cancer (NSCLC) with activating HER2 mutat...
Read More...
Sep 02, 2024
CAR T-Cell Therapies in Non-Hodgkin’s Lymphoma Treatment: A Revolutionary Approach
CAR-T cells offer a lasting benefit through a single treatment, sparing patients with high-risk conditions from the toxicity associated with salvage chemotherapy and autologous transplants. These approvals have changed the standard of care for patients who are either resistant to initial treatment or experience ear...
Read More...
Aug 30, 2024
NMIBC Treatment Paradigm Evolution: A Look At The Clinical Development Shift From High-Risk Bcg Unresponsive To Untapped High-Risk Bcg Naïve And Intermediate-Risk NMIBC
A high cure rate characterizes Non-muscle Invasive Bladder Cancer (NMIBC), yet there is a notable risk of recurrence and progression to muscle-invasive disease. Effective NMIBC management involves precise local resection, staging, and a risk-based approach with intravesical agents. The conventional gold standard fo...
Read More...
Aug 29, 2024
Insulet Corporation’s Omnipod® 5 Automated Insulin Delivery System Receives FDA Approval; FDA Gives Green Light to Expand Vericel’s MACI Arthro Label; SkinCure Oncology Announces New Study of Non-melanoma Skin Cancer Through Image-guided Superficial Radiation Therapy; SDC’s BYOD ePRO Solution Clinical Trial; PaceMate® Acquires Paceart Optima™ System; KARL STORZ Acquires a US-headquartered Company
FDA Cleared the Omnipod® 5 Automated Insulin Delivery System for Use in People with Type 2 Diabetes On August 26, 2024, Insulet Corporation, the global leader in tubeless insulin pump technology with its Omnipod® brand of products, announced that its groundbreaking Omnipod 5 Automated Insulin Delivery Syst...
Read More...