All Blogs
Oct 13, 2025
Digital Twins in the Healthcare Industry
Digital twins are advanced digital replicas of physical systems, processes, or entities that use real-time data, artificial intelligence (AI), machine learning (ML), and the Internet of Things (IoT) to simulate, monitor, and predict the behavior of their real-world counterparts. In healthcare, digital twins can rep...
Read More...
Jan 30, 2024
Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR T Cell Therapy Abecma for Multiple Myeloma; FDA Approves Dupixent for Eosinophilic Esophagitis; Juvena Receives FDA Orphan Drug Designation for JUV-161; European Commission Authorizes GSK’s Omjjara; ENHERTU Granted Priority Review in the US for for metastatic HER2-positive Solid Tumors
Merck’s KEYTRUDA Reduced the Risk of Death by 38% Versus Placebo as Adjuvant Therapy for Patients With Renal Cell Cancer (RCC) at an Increased Risk of Recurrence Following Nephrectomy Merck, also known as MSD beyond the United States and Canada, has revealed findings from the Phase III KEYNOTE-564 trial, which a...
Read More...
Jan 29, 2024
Atherosclerotic Cardiovascular Disease (ASCVD) Treatment Approaches: Beyond Statins
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of morbidity and mortality worldwide, encompassing conditions such as coronary artery disease and stroke. The growing occurrence of ASCVD, fueled by elevated risk factors, cardiovascular disease, and diabetes, is contributing to an expanding market f...
Read More...
Jan 25, 2024
FDA Breakthrough Device Designation to Pi-Cardia’s ShortCut; AbSolutions Med’s REBUILD Bioabsorbable Abdominal Wall Closure Device; AngioDynamics Announces FDA 510(k) Clearance of Auryon XL Radial Access Catheter; Enhatch Announces FDA Clearance for a TKA Patient-Specific Instrumentation System
Pi-Cardia Receives FDA Breakthrough Device Designation for ShortCut™ Pi-Cardia Ltd., a prominent player in advancing catheter-based leaflet modification solutions for heart valve treatment, revealed that its ShortCut™ device has attained Breakthrough Device Designation from the US Food and Drug Administration. S...
Read More...
Jan 26, 2024
AstraZeneca Strengthens Presence in PNH Treatment with Voydeya in Japan: World’s First Approval
A month following Novartis’ foray into the paroxysmal nocturnal hemoglobinuria (PNH) treatment domain, AstraZeneca strengthens its position in this field as its latest contender, Voydeya, secures a groundbreaking approval in Japan. Voydeya (danicopan), an innovative oral factor D inhibitor, has received approval fr...
Read More...
Jan 24, 2024
Airway Management Devices: Charting the Evolving Market Trends and Key Innovations
Technological evolution has significantly transformed Airway Management Devices in recent years, ushering in a new era of efficiency, precision, and patient safety. Advances in materials, design, and manufacturing processes have led to the development of more sophisticated devices. Moreover, the incorporation of hi...
Read More...
Jan 23, 2024
BMS, and Exelixis’s Opdivo + CABOMETYX in First-Line Advanced Renal Cell Carcinoma; AIRSUPRA Now Available as the First and Only FDA-approved Anti-inflammatory Rescue Option for Asthma; AstraZeneca’s Voydeya Receives First-ever Regulatory Approval; EMA Grants ODD to GC Biopharma’s Sanfilippo Syndrome (Type A) Treatment; FDA Approves NRx Pharma’s IND Application of NRX-101; FDA Fast Track Designation to Kyverna’s KYV-101
Opdivo in Combination with CABOMETYX Demonstrates Long-Term Survival Benefits After Four Years of Follow-Up in the CheckMate -9ER Trial in First-Line Advanced Renal Cell Carcinoma Bristol Myers Squibb and Exelixis, Inc. have released the four-year follow-up findings from the CheckMate -9ER trial, which investiga...
Read More...
Jan 22, 2024
Achondroplasia Treatment Space: Hunt for Potential Curative Therapies
Achondroplasia is a rare genetic disorder affecting bone growth, causing significant short stature, known as dwarfism. This condition is attributed to a fibroblast growth factor receptor 3 (FGFR3) gene mutation. The mutation results in an overactive FGFR3 gene, leading to a slowdown in bone formation within the car...
Read More...
Sep 04, 2024
Game-Changers in Non-Alcoholic Steatohepatitis (NASH) Treatment: Insights into Novel Drug Classes
Non-alcoholic steatohepatitis (NASH) has emerged as a global health concern, affecting millions of individuals worldwide. NASH is a progressive liver disease characterized by inflammation and liver cell damage, often resulting from the accumulation of fat in the liver. In 2023, there were an estimated 42 million pr...
Read More...
Jan 18, 2024
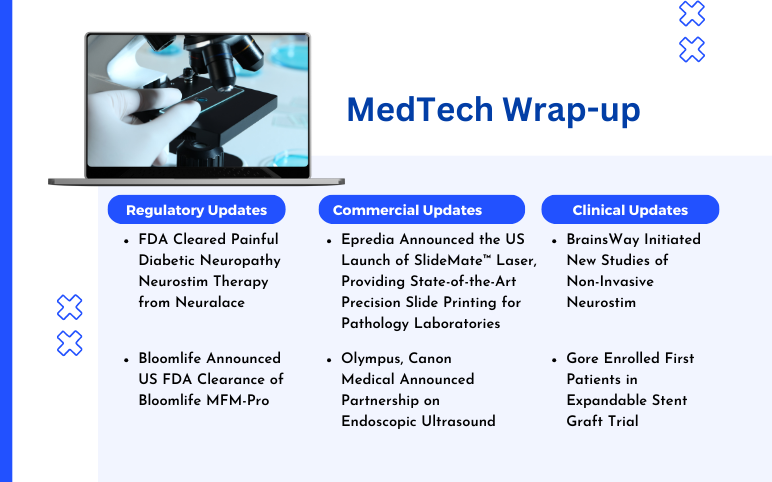
Epredia Announced the US Launch of SlideMate Laser; Olympus, Canon Medical Announced Partnership; FDA Cleared Painful Diabetic Neuropathy Neurostim Therapy from Neuralace; FDA Clearance to Bloomlife’s Bloomlife MFM-Pro; BrainsWay Initiated New Studies of Non-Invasive Neurostim; Gore Enrolled First Patients in Expandable Stent Graft Trial
Epredia Announced the US Launch of SlideMate™ Laser, Providing State-of-the-Art Precision Slide Printing for Pathology Laboratories On January 11, 2024, Epredia, a global leader in precision cancer diagnostics, announced that the company launched US sales of SlideMate™ Laser, the newest addition to the company’s...
Read More...