All Blogs
Sep 12, 2023
BMS’s LPA1 Antagonist; Alnylam’s KARDIA-1 Phase 2 Study; Day One Biopharma Sought FDA Approval for Tovorafenib; EMA Orphan Drug Designation to MaaT Pharma’s MaaT033; Lundbeck and Otsuka Announce Topline Results from Two Phase III Trials of Brexpiprazole + Sertraline; Phase III CheckMate – 227 Trial Show Durable, Long-Term Survival with Opdivo Plus Yervoy
Bristol Myers Squibb’s Investigational LPA1 Antagonist Reduces Rate of Lung Function Decline in Progressive Pulmonary Fibrosis Cohort of Phase II Study BMS-986278, a potential first-in-class oral lysophosphatidic acid receptor 1 (LPA1) antagonist, was studied in patients with progressive pulmonary fibrosis (PPF)...
Read More...
Jul 25, 2024
BMS Vs. Janssen: Which Company Will Dominate The Multiple Myeloma Treatment Market This Decade?
Over the past several years, multiple myeloma treatment options have expanded widely for patients, resulting in significantly improved outcomes. The FDA approved around 16 new agents and 30 treatment regimens, transforming the multiple myeloma treatment paradigm for patients with newly diagnosed and relapsed/refrac...
Read More...
May 09, 2025
ADCs in Lung Cancer Treatment: ENHERTU’s Rise, HER3 & TROP-2 Challenges, and What’s Next in the Pipeline
Lung cancer remains the leading cause of cancer-related death in the United States; approximately 85% of lung cancers are non-small cell lung cancer (NSCLC), with ~530K cases in the 7MM. The main subtypes of NSCLC are adenocarcinoma (~57%), squamous cell carcinoma (~26%), and large cell carcinoma (~2%). Despite adv...
Read More...
Sep 07, 2023
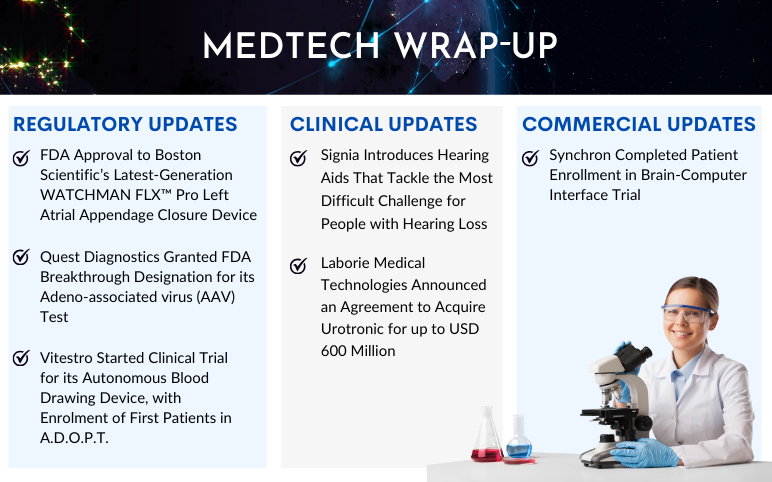
Boston Scientific’s WATCHMAN FLX™ Pro; Quest Diagnostics’s AAV Test; Vitestro Started A.D.O.P.T. Clinical Trial; Signia Introduces Hearing Aids; Laborie Medical to Acquire Urotronic; Synchron’s Brain-Computer Interface Trial
Quest Diagnostics Granted FDA Breakthrough Designation for its Adeno-associated virus (AAV) Test On August 30, 2023, Quest Diagnostics announced that its AAVrh74 ELISA assay (CDx) has been granted Breakthrough Device Designation from the US Food and Drug Administration (FDA). The enzyme-linked immunosor...
Read More...
Sep 06, 2023
Revolutionizing Pain Management: The Growth in Innovative Devices and Rise of Market
Chronic pain is an unwelcome companion in the journey of life and can significantly impact the quality of life. Being in pain serves as a protective mechanism that warns us when something is wrong with the concerned body part, and is a natural component of the human experience. But for millions of people around the...
Read More...
Sep 05, 2023
Daiichi Sankyo’s Trastuzumab Deruxtecan; ODD to Bexmarilimab for AML; Roche’ Alecensa; Ergomed Aims To Go Private; Tagraxofusp Receives ODD in Japan for BPCDN; FDA Fast Track Designation to Abliva’s KL1333
FDA Grants Breakthrough Therapy Designations to Trastuzumab Deruxtecan for HER2+ Solid Tumors, Including mCRC ENHERTU® (fam-trastuzumab deruxtecan-nxki) has been granted two additional Breakthrough Therapy Designations (BTDs) in the United States for the treatment of adult patients with unresectable or metastati...
Read More...
Sep 04, 2023
Fabry Disease – A Market Perspective On The Emerging Pipeline
Fabry disease is a rare hereditary lysosomal storage disorder that is caused by mutation in the GLA gene located on the X chromosome. The defect leads to the deficiency of an enzyme called alpha-galactosidase A, responsible for the breakdown of globotriaosylceramide (Gb3). The accumulation of the fatty substance le...
Read More...
Sep 24, 2024
Unleashing the Potential: CD38 Directed Therapies Revolutionize Multiple Myeloma Treatment
The landscape of multiple myeloma therapies has been revolutionized by the introduction of cutting-edge monoclonal antibodies (mAbs) targeting CD38. Among the standout advancements are DARZALEX (daratumumab) and SARCLISA (isatuximab-irfc), which have emerged as game-changers in the treatment of multiple myeloma. Th...
Read More...
Aug 31, 2023
GE HealthCare Launched CardioVisio; J&J’s Elita Laser Correction Platform; ICU Medical’s Plum Duo Infusion Pump and LifeShield Infusion Safety Software Update; Medtronic’s Inceptiv SCS with Closed-Loop Sensing to Treat Chronic Pain; Boston Scientific’s FARAPULSE Pulsed Field Ablation System; Epineuron’s REGAIN Trial
GE HealthCare Launched CardioVisio for Atrial Fibrillation, a Digital, Patient-Centric Clinical Decision Support Tool to Enable Precision Care On August 24, 2023, GE HealthCare launched CardioVisio for Atrial Fibrillation (AFib), a digital tool designed to assist clinicians in visualizing longitudinal data relev...
Read More...
Aug 30, 2023
Harnessing Light for Health: Evaluating the Growing Demand for Light Therapy
In recent years, the rapid development of technology and innovation has sped up the transformation of the healthcare and well-being industry. Among the various emerging and evolving trends, Light Therapy is one of the key technologies gaining immense attention among the users. Light Therapy is an ancient practice, ...
Read More...