Opioid-Induced Respiratory Depression: Unveiling the Silent Threat
Dec 22, 2023
Table of Contents
In recent years, the misuse and abuse of opioids have become a growing concern worldwide, with devastating consequences for public health. While the immediate dangers of opioid overdose are well-documented, a silent and potentially lethal complication often lurks in the shadows—opioid-induced respiratory depression.
Opioid-induced respiratory depression (OIRD) is a serious condition resulting from decreased respiratory drive, sedation, and upper airway obstruction caused by opioids. If not identified and treated promptly, OIRD can be fatal. Comorbidities like sleep apnea, renal and hepatic diseases, COPD, cardiac issues, and obesity elevate the risk of OIRD. Combining opioids with benzodiazepines increases the danger of respiratory arrest, as their depressant effects on the respiratory system are additive.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Unlocking New Avenues in KRAS-Driven Cancer Research Beyond G12C
- How Artificial Intelligence is adding value to the Healthcare sector
- FDA Breakthrough Device Designation to Pi-Cardia’s ShortCut; AbSolutions Med’s REBUILD Bioabsorba...
- The Business Cocktail
- ATS 2023 Updates: Dupixent – A Ray of Hope For Moderate To Severe Chronic Obstructive Pulmo...
Opioid-induced respiratory depression manifests as a decreased respiratory rate (less than 8 breaths/min), potentially leading to low oxygen saturation (<85%), elevated arterial pCO2 levels, and reduced ventilatory response to hypoxia and hypercapnia, especially in severe cases. As per DelveInsight’s Analysis, it is estimated that the age-specific cases of OIRD are the highest in >40–50 years and are expected to increase during the forecast period (2023–2032).
The Opioid Epidemic: A Grave Public Health Crisis
The opioid epidemic represents a grave public health crisis that has swept across nations, leaving devastation in its wake. Characterized by the widespread misuse and addiction to prescription opioids, as well as illicit substances like heroin, this crisis has reached alarming proportions, claiming countless lives and inflicting profound societal consequences. Originating from the overprescription of painkillers, the epidemic has spiraled into a complex web of addiction, overdoses, and shattered communities. The toll is not only measured in the staggering number of lives lost but also in the shattered families, strained healthcare systems, and the strain on law enforcement and social services.
DelveInsight’s analysis reveals that the overall prevalent cases of opioid use disorder in the 7MM were reported as ~4 million in 2022. Within this, the prevalent cases of opioid use disorder patients in the United States specifically were identified to be ~53% in the same year.
The opioid epidemic is a multifaceted challenge that demands a comprehensive and compassionate response, combining efforts from healthcare professionals, policymakers, law enforcement, and the community at large to address the root causes, provide effective treatment, and prevent further tragedies. It stands as a stark reminder of the urgent need for proactive measures to tackle addiction, destigmatize mental health, and reshape healthcare policies for the well-being of society as a whole. Opioid-induced respiratory depression emerges as a deadly consequence, demanding immediate attention.
The Role of Naloxone in Opioid-Induced Respiratory Depression Treatment
Naloxone infusion stands as the exclusive remedy for reversing opioid-induced respiratory depression. The success of naloxone hinges on its pharmacological characteristics and those specific to the opioid requiring reversal. The reversal process becomes more intricate when dealing with high-affinity opioids, given the abbreviated elimination and biophase equilibration half-lives of naloxone, along with the swift receptor kinetics. Effective reversal of high-affinity opioids necessitates elevated naloxone concentrations and/or a continuous infusion, in contrast to opioids with lower receptor affinity.
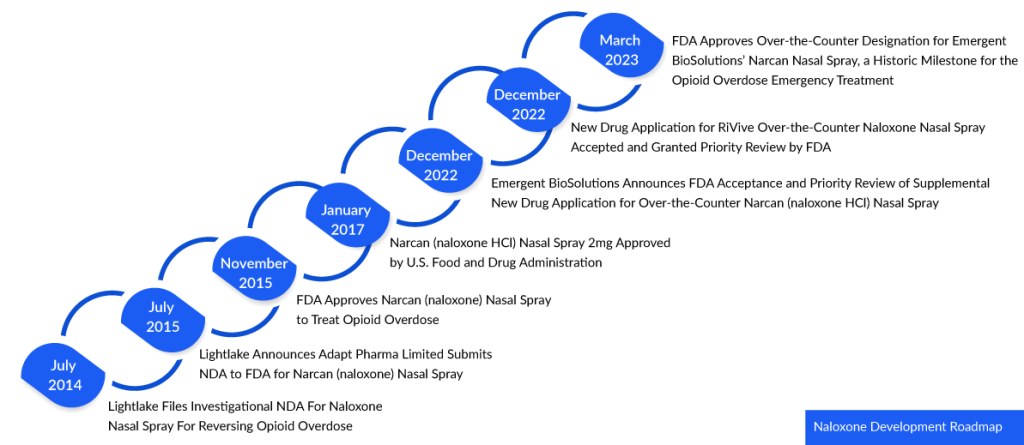
In 1971, Naloxone received its initial approval in the United States under the trade name NARCAN. Initially available in injectable forms for intravenous (IV), intramuscular (IM), or subcutaneous (SC) administration, it became a vital tool for medical professionals and first responders. Recently in March 2023, a historic milestone was reached as the FDA granted approval for Emergent BioSolutions’ NARCAN nasal spray, allowing it to be obtained over-the-counter (OTC) without a prescription. This approval marked the first time a naloxone product was cleared for nonprescription use, expanding its accessibility and potential impact.
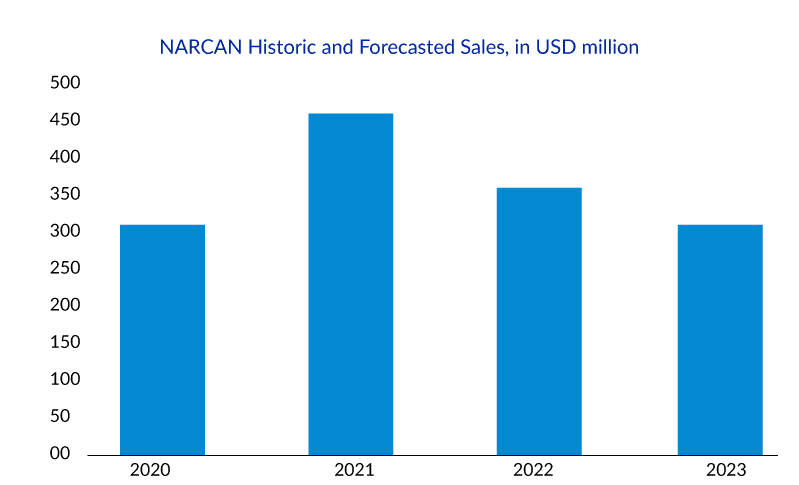
NARCAN Nasal Spray was specifically designed for community use, and since its introduction in 2016, over 44 million doses have been distributed. However, with the introduction of generic alternatives in December 2021, NARCAN sales experienced a decrease from USD 434.3 million in 2021 to USD 373.3 million in 2022. Faced with declining revenue and the possibility of competition from Harm Reduction Therapeutics’ RiVive, Emergent BioSolutions sought and obtained approval for the over-the-counter (OTC) availability of NARCAN Nasal Spray.
Despite naloxone’s efficacy in reversing opioid toxicity, the need for more potent and enduring reversal agents persists. Crafting more user-friendly formulations could greatly improve their widespread use. Instead of solely reacting to overdoses, a shift towards preventive strategies—such as abuse-deterrent opioid formulations or medications that selectively address respiratory depression while maintaining pain control—is essential. Customized opioid-induced respiratory depression treatment plans based on individual patient characteristics, including genetics and metabolism, offer promise in enhancing outcomes and minimizing adverse events associated with opioid use.
Promising Therapies for Opioid-Induced Respiratory Depression Treatment on the Horizon
In the global opioid-induced respiratory depression treatment landscape, companies are fervently driving innovation in the realm of treatment therapies, witnessing considerable success in their pursuits. Among the emerging opioid-induced respiratory depression treatment market leaders, Atelerix Life Sciences and Enalare Therapeutics stand out as contributors to the development of drugs for opioid-induced respiratory depression treatment.
Atelerix Life Sciences has pioneered ATLX-0199 (D-Cysteine diME) as its flagship product. This prodrug, administered via IV injection, undergoes conversion within red blood cells, ultimately yielding the powerful active respiratory stimulant SNO-CysME. Upon exiting red blood cells and entering glomus cells, SNO-CysME effectively binds to crucial voltage-gated potassium channels in the carotid body, initiating neural signals that augment respiration and counteract opioid-induced respiratory depression.
Compelling pre-clinical proof of concept has been demonstrated through in-vitro and in-vivo studies, revealing that ATLX-0199 enhances minute ventilation and successfully mitigates opioid-induced respiratory depression. In specific tests with conscious rats administered 10 mg/kg IV morphine, ATLX-0199 (D-Cysteine diME) reverses respiratory depression and corrects ventilation/perfusion (V/Q) mismatch. Importantly, ATLX-0199 does not compromise the analgesic efficacy of morphine in freely moving rats. The late pre-clinical stages for OIRD treatment are currently underway.
Enalare Therapeutics is making significant strides with its lead drug candidate, ENA-001, a newly patented compound set to commence additional clinical trials in the fourth quarter of 2021. Distinguished by its novel mechanism of action and proven safety and efficacy, ENA-001 holds immense promise for improving the quality of life for individuals dealing with serious conditions such as drug overdose, post-operative respiratory depression, and apnea of prematurity. This groundbreaking drug opens up new avenues for treatment, providing healthcare professionals, emergency responders, and caregivers with effective options for addressing respiratory depression across diverse settings. Enalare Therapeutics’ commitment to advancing ENA-001 is underscored by their expanded partnership with BARDA, announced in September 2022, focusing on its development as an emergency treatment for opioid-induced respiratory depression.
The anticipated launch of these therapies will definitely create a positive impact on the opioid-induced respiratory depression market in the coming years.
Innovations Shaping the Future of Opioid-Induced Respiratory Depression Treatment
The future of treating opioid-induced respiratory depression holds promise as advancements in medical science and technology converge to address this critical healthcare concern. With opioid use on the rise for pain management, the risk of respiratory depression, a potentially life-threatening side effect, has become a focal point of research and innovation. Emerging technologies such as smart monitoring devices and artificial intelligence are playing pivotal roles in the early detection of respiratory depression, allowing for timely intervention. Novel pharmacological approaches, including opioid receptor modulators and respiratory stimulants, are being explored to mitigate the adverse effects of opioids on respiratory function.
Additionally, personalized medicine and genetic profiling are opening avenues for tailoring treatment strategies to individual patient characteristics, enhancing both efficacy and safety. As interdisciplinary collaborations between clinicians, researchers, and technology experts intensify, the future holds the promise of not only effectively treating opioid-induced respiratory depression but also transforming the landscape of pain management to minimize associated risks. Through these innovative approaches, the healthcare community is working towards a safer and more personalized future for individuals navigating the complexities of opioid-based therapies.

Downloads
Article in PDF
Recent Articles
- Evaluating the Essential Factors Building the Value-Based Healthcare Model
- The Automated Eye: How AI-Driven Diagnostics are Reshaping the Future of Medical Imaging
- DUPIXENT Receives First-Ever Biologic Approval for COPD: Adds Another Jewel in its Crown
- CTEXLI Approved for Cerebrotendinous Xanthomatosis; SIGX1094 Wins Fast Track for Diffuse Gastric ...
- Is Air Pollution a driver in COPD Treatment Market?