All Blogs
Sep 25, 2023
Orchard’s OTL-200 to Enter US Metachromatic Leukodystrophy Treatment Space After EU
Orchard Therapeutics’ Biologics License Application for OTL-200, a gene therapy under investigation for metachromatic leukodystrophy treatment, has been accepted by the FDA. This rare disease treatment received approval in Europe back in 2020. Notably, the FDA has granted Orchard’s application Priority Review statu...
Read More...
Sep 22, 2023
Myelofibrosis Treatment Market Heats Up with GSK’s Momelotinib Entry
On September 15, the FDA authorized GSK’s oral medication momelotinib, now known as Ojjaara, for the treatment of myelofibrosis in adults with anemia. Myelofibrosis is a disorder where normal bone marrow tissue is slowly substituted by fibrous, scar-like material. It is categorized as a form of chronic leukemia and...
Read More...
Sep 21, 2023
Zepp Health Launched OTC Hearing Aids; Neoss Launched NeoScan 2000; FDA Clearance for AI-Assisted Sleep Monitoring Device Dreem 3S; Tasso Received CE Mark Certification for Tasso+; Neuralink’s First-in-human BCI trial; Creative Medical to Initiate a Phase I/II Clinical Trial of StemSpine
Zepp Health Launched a New Line of OTC Hearing Aids for its Zepp Clarity Brand On September 19, 2023, Zepp Clarity, a smart hearing solutions brand owned by Zepp Health, a health technology company, announced the launch of Zepp Clarity Pixie, a next-generation premium hearing solution. The Pixie, which ...
Read More...
Sep 20, 2023
Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs
Sleep Aids Market: Examining Cutting-Edge Technologies and Scientific Breakthroughs Sleep can be burdensome when it disrupts daily routines, requires a significant time commitment, and is marred by sleep disorders or disturbances that affect overall well-being. In today's fast-paced world, where stress, anxiety,...
Read More...
Sep 19, 2023
FDA Approves Ojjaara for Myelofibrosis; EMA Grants PRIME Designation to Iopofosine I-131; EBGLYSS Receives Positive CHMP Opinion; FDA Accepts Resmetirom NDA; FDA Fast Track Designation to KT-333 for PTCL; RedHill Announces FDA sNDA Approval for Talicia®
EBGLYSS Receives Positive CHMP Opinion for Moderate-to-Severe Atopic Dermatitis Almirall S.A. announced that the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion recommending the marketing authorization of EBGLYSS (lebrikizumab) for the treatment o...
Read More...
Sep 18, 2023
A Turning Point: INGREZZA’s Impact on Huntington’s Disease Treatment and the Rise of Novel Therapies
Huntington’s disease is an incurable, rare genetic, progressive neurodegenerative disorder. According to the National Organization for Rare Disorders (NORD), about 30,000 people in the United States have Huntington’s disease, and another 200,000 are at risk of developing the condition. As per DelveInsight analy...
Read More...
Sep 15, 2023
Myelodysplastic Syndrome Treatment Market: Unveiling the Robust Pipeline
The number of people diagnosed with myelodysplastic syndrome in the US each year is unknown. However, some estimates have put this number at about 10,000, while other estimates have been much higher. Moreover, myelodysplastic syndrome is uncommon before age 50, and the risk increases as a person gets older. It is a...
Read More...
Sep 14, 2023
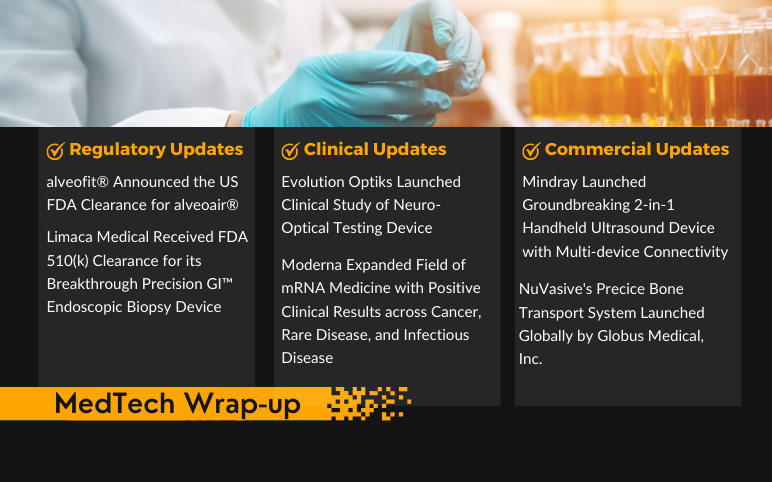
Mindray’s 2-in-1 Handheld Ultrasound Device; NuVasive’s Precice Bone Transport System; FDA for alveofit’s alveoair; FDA 510(k) Clearance Limaca’s Precision GI™ Endoscopic Biopsy Device; Evolution Optiks’s Neuro-Optical Testing Device; Moderna Expanded Field of mRNA Medicine
Mindray Launched Groundbreaking 2-in-1 Handheld Ultrasound Device with Multi-device Connectivity On September 7, 2023, Mindray, a global leader and developer of healthcare solutions and technologies in ultrasound, patient monitoring, and anesthesia announced the release of its new imaging solution, the TE ...
Read More...
Sep 13, 2023
Biosurgery: Advancing Medicine through Biologically Derived Solutions
In recent years, the healthcare and medical devices market has undergone a remarkable transformation, largely fueled by advancements in technology and growth in innovation. One of the major standout developments in the healthcare industry is the rise of the biosurgery market and the related product demand, whic...
Read More...
Sep 12, 2023
BMS’s LPA1 Antagonist; Alnylam’s KARDIA-1 Phase 2 Study; Day One Biopharma Sought FDA Approval for Tovorafenib; EMA Orphan Drug Designation to MaaT Pharma’s MaaT033; Lundbeck and Otsuka Announce Topline Results from Two Phase III Trials of Brexpiprazole + Sertraline; Phase III CheckMate – 227 Trial Show Durable, Long-Term Survival with Opdivo Plus Yervoy
Bristol Myers Squibb’s Investigational LPA1 Antagonist Reduces Rate of Lung Function Decline in Progressive Pulmonary Fibrosis Cohort of Phase II Study BMS-986278, a potential first-in-class oral lysophosphatidic acid receptor 1 (LPA1) antagonist, was studied in patients with progressive pulmonary fibrosis (PPF)...
Read More...