Exploring the Current Alzheimer’s Disease Drug Development Pipeline
Feb 12, 2025
Table of Contents
Alzheimer’s disease continues to pose a significant challenge to global healthcare systems, with the 7MM reporting approximately 16 million diagnosed prevalent cases in 2023. This rising AD prevalence highlights an urgent need for innovative treatments to mitigate the growing disease burden on both patients and caregivers. The aging global population, coupled with the increasing Alzheimer’s disease diagnosis rate, makes it a critical focus for the pharmaceutical industry, driving robust research and development efforts.
In the United States, the disease disproportionately affects females, with 4.6 million women diagnosed in 2023 compared to 2.3 million men. This gender disparity is expected to widen by 2034, as Alzheimer’s becomes an even greater public health concern. DelveInsight’s Alzheimer’s disease epidemiology-based market report projects a continued rise in the overall diagnosed prevalent cases of Alzheimer’s disease in the coming years, reflecting an urgent need for effective therapeutic solutions.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- STRATA Skin Sciences’ XTRAC Momentum™ 1.0 Device Approved in Japan; eCential Robotics Received FD...
- Notizia
- Gene Therapy’s Emergence: The “New” approach for Huntington’s disease
- New Asundexian Phase III Study Result; Zibotentan/Dapagliflozin Combination Demonstrated Signific...
- The Growing Burden Of Neurodegenerative Disorders
The need for novel, disease-modifying therapies has never been more pressing, and this demand is driving the exploration of cutting-edge treatments, from amyloid-targeting agents to more comprehensive neuroprotective approaches.
With a dynamic and evolving drug pipeline, pharmaceutical companies are increasingly focused on developing therapies that slow the disease’s progression and improve patient quality of life. As we look to the future, new drug development, advanced biomarkers, and clinical trial strategies are expected to play pivotal roles in transforming the Alzheimer’s disease landscape, offering hope to millions affected worldwide.
Current Landscape of Alzheimer’s Disease Drug Development
The current landscape of Alzheimer’s disease drug development is characterized by a combination of approved therapies and ongoing research into new treatment options. In 2023, the AD market in the 7MM was valued at approximately USD 3.6 billion, with a range of therapies contributing to this figure. The approved treatments primarily focus on managing symptoms rather than addressing the underlying disease pathology. These include cholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, which help improve cognitive function in the short term, and memantine, an NMDA receptor antagonist, which aids in preventing excess glutamate activity in the brain.
Despite these treatment options, none of the current therapies can halt the progression of Alzheimer’s disease or reverse its underlying pathology. The focus remains on symptom management, with patients often relying on these medications to temporarily slow cognitive decline.
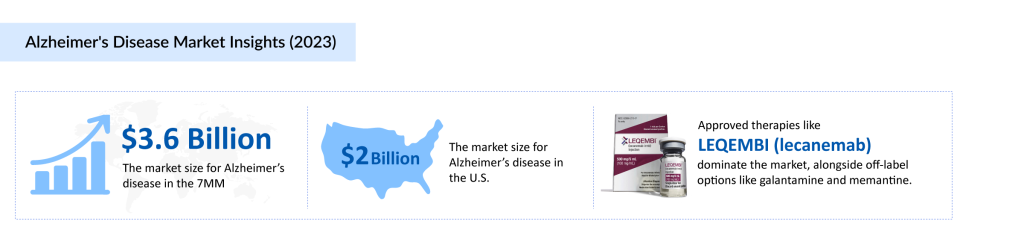
Recent advancements in the field have introduced the use of anti-amyloid monoclonal antibodies, such as LEQEMBI (lecanemab), which targets amyloid plaques in the brain. The hope is that these therapies can slow the cognitive decline associated with Alzheimer’s by reducing the accumulation of amyloid-beta proteins. However, these new treatments have faced regulatory and clinical scrutiny concerning their efficacy and safety, particularly regarding their ability to significantly alter disease progression.
The treatment landscape also incorporates non-pharmacological interventions, which play a crucial role in supporting patients’ overall well-being. Cognitive therapies, physical exercise, and lifestyle modifications are vital components of care that contribute to the mental and physical health of Alzheimer’s patients.
Looking ahead, the AD drug development pipeline remains dynamic, with several emerging therapies poised to enter the market in the coming years. These novel treatments, along with ongoing research into disease-modifying drugs, are expected to reshape the Alzheimer’s treatment landscape, offering patients and their families hope for more effective management and potentially disease-modifying therapies.
Key Targets in Alzheimer’s Disease Drug Development
Alzheimer’s disease drug development focuses on multiple biological targets, aiming to slow disease progression and alleviate symptoms. The primary targets include amyloid beta (Aβ) plaques and tau proteins, which are hallmark pathological features of AD. Additionally, researchers are exploring other mechanisms, such as neurotransmitter modulation, inflammation, and oxidative stres,s to develop more effective therapies.
Amyloid Beta (Aβ) as a Target
Aβ accumulation in the brain is a central feature of AD pathology, leading to plaque formation and neuronal toxicity. Targeting Aβ aims to reduce or clear these plaques to slow cognitive decline.
- Aducanumab (Aduhelm®) – FDA-approved for early AD with mild cognitive impairment (MCI), targeting aggregated Aβ to reduce plaque buildup.
- Lecanemab (Leqembi®) – FDA-approved for early AD, shown to reduce amyloid plaques and slow disease progression.
- Donanemab – Recently FDA-approved for early AD, focusing on clearing existing amyloid plaques to slow cognitive decline.
Tau Protein as a Target
Tau proteins form neurofibrillary tangles (NFTs) in AD, disrupting neuronal function. Targeting tau aggregation may help prevent neurodegeneration.
- Tau aggregation inhibitors – Designed to prevent or disrupt the formation of toxic tau oligomers and NFTs.
- Anti-tau monoclonal antibodies – Aiming to reduce tau pathology and slow disease progression.
Other Emerging Targets in AD Drug Development
Beyond amyloid and tau, researchers are exploring additional pathways involved in AD progression:
- Apolipoprotein E (ApoE) – A genetic risk factor influencing amyloid deposition and cholesterol metabolism in the brain.
- Neurotransmitter receptors – Drugs targeting cholinergic and glutamatergic systems aim to improve cognitive function and neuronal communication.
- Inflammation – Chronic neuroinflammation contributes to AD progression, making anti-inflammatory strategies a promising avenue.
- Synaptic function – Enhancing synaptic plasticity and neuroprotection may help preserve cognitive abilities.
- Oxidative stress – Addressing free radical damage and mitochondrial dysfunction could mitigate neuronal loss.
- Cholinergic deficits – Acetylcholinesterase inhibitors, such as donepezil, rivastigmine, and galantamine, help boost acetylcholine levels to temporarily improve cognitive function.
As research evolves, multi-targeted approaches integrating amyloid clearance, tau modulation, and neuroprotection are expected to play a crucial role in the next generation of AD therapies.
Challenges in AD Drug Development
Alzheimer’s disease drug development is a formidable challenge, marked by high failure rates, complex disease mechanisms, and the lack of disease-modifying treatments. Despite years of research, most therapies have focused on symptom management rather than stopping or reversing neurodegeneration.
One of the biggest hurdles is the multifactorial nature of AD, involving amyloid plaques, tau tangles, neuroinflammation, and synaptic dysfunction. Many therapies have targeted amyloid beta, but mixed clinical outcomes suggest a need for multi-targeted approaches. Additionally, the slow progression of the disease makes clinical trials lengthy and costly, while the lack of clear biomarkers complicates early diagnosis and treatment efficacy assessments.
Even with recent FDA-approved therapies like aducanumab and lecanemab, concerns around safety, effectiveness, and accessibility remain. Moving forward, success in AD drug development will require combination therapies, early detection strategies, and innovative clinical trial designs to change the trajectory of this devastating disease finally.
Recent Advances and Success Stories
The field of Alzheimer’s disease research has witnessed remarkable advancements in recent years, with breakthroughs in diagnostics, novel drug approvals, and promising clinical trial results. These developments reshape the treatment landscape and provide new hope for patients and caregivers.
Breakthroughs in Early Diagnosis
Early detection of Alzheimer’s is crucial in improving patient outcomes, and Spear Bio Inc.’s recent innovation in blood-based diagnostics marks a significant leap forward. In January 2025, its pTau 217 blood test received Breakthrough Device Designation from the FDA, recognizing its potential to address the critical unmet need for early and accurate AD diagnosis.
Expanding Treatment Options
The anti-amyloid therapy landscape continues to evolve, with BioArctic AB and Eisai achieving a key regulatory milestone in January 2025. The FDA accepted a Biologics License Application (BLA) for the Leqembi subcutaneous autoinjector (SC-AI), a more convenient weekly maintenance dosing option for patients with early Alzheimer’s. This innovation aims to enhance patient adherence and accessibility.
Similarly, Eli Lilly’s Kisunla (donanemab) is making strides on the global stage. In December 2024, it secured regulatory approval in China, opening doors for expanded patient access in the world’s second-largest pharmaceutical market. Following this, in September 2024, Kisunla also received approval in Japan, solidifying its presence in major international markets.
New Targets and Emerging Therapies
While amyloid beta remains a central target, phosphorylated tau-directed therapies are also gaining traction. In January 2025, the FDA granted Fast Track designation to posdinemab, a monoclonal antibody under investigation in the Phase 2b AuTonomy study for early Alzheimer’s treatment.
At the same time, Axsome Therapeutics is advancing AXS-05, a combination of dextromethorphan and bupropion, for Alzheimer’s agitation. Despite mixed late-phase results, the company remains committed to pursuing FDA approval, recognizing the urgent need for better symptom management therapies.
Repurposed Alzheimer’s Drugs in the Pipeline Showing Promise
An unexpected breakthrough emerged from real-world studies on semaglutide, a diabetes drug from Novo Nordisk. In October 2024, a study published in Alzheimer’s and Dementia revealed that semaglutide was associated with a 40% to 70% reduction in the risk of AD diagnosis in patients with type 2 diabetes. This follows previous studies linking semaglutide to lower dementia risk, reinforcing its potential for future clinical development in Alzheimer’s treatment.
Regulatory Setbacks and Challenges
Despite these advancements, cost and accessibility remain key hurdles. In October 2024, Eli Lilly’s Kisunla faced a setback in the UK, where it was deemed too expensive for widespread use by regulatory authorities, despite its approval by the Medicines and Healthcare products Regulatory Agency (MHRA). This highlights the ongoing challenge of balancing innovation with affordability.
Path to the Future
With these groundbreaking developments in diagnostics, drug approvals, and emerging therapies, the Alzheimer’s landscape is evolving faster than ever before. While challenges remain, the industry is making significant strides toward early detection, better treatment options, and ultimately, a future where Alzheimer’s can be managed more effectively—or even cured.

Conclusion
The Alzheimer’s disease pipeline is undergoing a transformational shift driven by cutting-edge research, groundbreaking diagnostics, and an expanding arsenal of Alzheimer’s disease therapies. From next-generation anti-amyloid treatments to novel tau-targeting and neuroprotective strategies, the Alzheimer’s disease therapeutic landscape is more dynamic than ever before. However, the road ahead is not without obstacles—high drug development failure rates, regulatory scrutiny, and cost barriers continue to challenge progress. Yet, the momentum is undeniable. With emerging biomarkers enabling earlier diagnosis, innovative clinical trial designs accelerating drug approval, and multi-targeted approaches unlocking new possibilities, the future of Alzheimer’s treatment is filled with renewed hope. As science pushes the boundaries, we move closer to a world where Alzheimer’s is no longer a devastating inevitability but a condition that can be effectively treated—or even prevented.

FAQs
In 2023, there were about 16 million diagnosed cases of Alzheimer’s across the 7MM, and this number is expected to rise sharply by 2034. Women already make up the majority of cases, and the gender gap is projected to widen due to longer female life expectancy and higher susceptibility.
The Alzheimer’s market was valued at USD 3.6 billion in 2023, with revenues mostly from symptomatic drugs. With the arrival of anti-amyloid therapies like LEQEMBI and KISUNLA, the market is entering a growth phase, driven by demand for disease-modifying treatments.
Anti-amyloid monoclonal antibodies such as Aduhelm, Leqembi, and Kisunla are shifting treatment from symptom relief toward slowing disease progression. While safety and cost remain challenges, these drugs represent the first real step toward modifying the course of Alzheimer’s.
Early detection is vital, and blood-based biomarkers like Spear Bio’s pTau 217 test, granted FDA Breakthrough Device status in January 2025, are game-changers. These tests are more accessible than PET scans and could allow treatment to begin earlier in the disease course.
Promising late-stage candidates include posdinemab, an anti-tau antibody given FDA Fast Track status in January 2025, and the Leqembi autoinjector under FDA review. Other therapies target inflammation, synaptic health, and neuroprotection, signaling a broader, multi-targeted future.
Yes, semaglutide, a diabetes drug, showed a 40–70% reduction in Alzheimer’s risk in type 2 diabetes patients, according to an October 2024 study. If proven in trials, such repurposed drugs could accelerate access to affordable new treatment options.
Alzheimer’s trials are long, costly, and often end in failure due to the disease’s complexity. Even approved drugs face barriers such as high costs, safety risks, and regulatory pushback, underscoring the need for biomarkers and combination therapies.
Downloads
Article in PDF
Recent Articles
- FDA Approves Sanofi/Regeneron’s DUPIXENT as First New CSU Therapy in Over a Decade; Gilead’s TROD...
- Boehringer Ingelheim’s Diabetic Macular Ischemia Study; Novartis’ Mariana Oncology Acquisition; A...
- Outbreak of Companies Challenging New Pathways for Treatment of Pulmonary Arterial Hypertension
- Moderna’s Phase III Trials for Dual Influenza and COVID-19 Vaccine; Almirall’s Klisyri FDA Approv...
- Laborie’s Enhanced Gastrointestinal Diagnostics; Carestream’s Image Suite MR 10 Software Launch; ...



