Will Cell Therapy Bridge the Gap in Bullous Keratopathy Treatment Space?
Aug 04, 2023
Table of Contents
Corneal blindness is the world’s fourth major cause of blindness. The two most common bases for corneal blindness are trauma, often associated with eye surgery and genetic abnormalities such as Fuchs dystrophy or bullous keratopathy. Bullous keratopathy, also known as edematous keratopathy, is a severe eye disorder that results from damage to the corneal endothelium, a monolayer of cells located on the posterior surface of the cornea. The corneal endothelial cells have focal tight junctions with sodium/potassium ATPase to help maintain the cornea in a dehydrated state. Due to endothelial dysfunction, aqueous humor enters the cornea, particularly the most superficial layers at first, which are formed by microvesicles or bullae, hence the name. When corneal edema develops, the cornea loses transparency, compromising on vision.
Bullous keratopathy after cataract removal is referred to as pseudophakic bullous keratopathy (PBK) if there is an intraocular lens implant present, and aphakic bullous keratopathy (ABK) if there is no intraocular lens implant present.
Downloads
Click Here To Get the Article in PDF
Recent Articles
Bullous keratopathy can result from various underlying causes, including eye trauma, cataract surgery, glaucoma surgery, trabeculectomy, laser iridotomy, or chronic conditions like Fuchs’ dystrophy. A National Survey states that bullous keratopathy may occur in around 1–2% of patients undergoing cataract surgery, a significant number considering that approximately 10 million patients undergo cataract surgery worldwide each year. Glaucoma surgery, laser, and Fuchs dystrophy are further causes of bullous keratopathy. As per the Delveinsight analysis in 2022, there were approximately 78K bullous keratopathy cases in the US.
Elderly people are the ones who most frequently develop bullous keratopathy. Determining the precise cause can sometimes be difficult, especially in cases where multiple factors contribute to the development of bullous keratopathy. Early detection and prevention strategies are crucial in managing bullous keratopathy. Initially, a blurred vision in the morning is observed; subsequently, this sensation of blurred vision extends throughout the day, and in later stages, cornea vesicles or bullae start to appear, which finally break, causing intense pain to the patient. This chronic pain can lead to permanent vision loss if not treated.
Current challenges in the diagnosis of bullous keratopathy
Bullous keratopathy, being a complex disease has an inherent disadvantage when it comes to its drug development. Its small population size creates logistical, regulatory, and fiscal roadblocks to bullous keratopathy clinical trials. It has been difficult for researchers to establish a concrete and curative therapy. Usually, patient histories and responses in bullous keratopathy clinical trials across regions provide safety and efficacy insights into a drug which is further used for better disease management. In the case of bullous keratopathy, there are limited trials being conducted with challenges reaching thresholds of clinical significance. This makes understanding of disease trajectory an uphill task.
The symptoms of bullous keratopathy, such as blurred vision and ocular pain, make it challenging for an ophthalmologist to distinguish it from other corneal disorders like corneal dystrophies, corneal infections, or corneal edema. Thus, an accurate and prompt diagnosis is critical. However, clinical bullous keratopathy symptoms alone may not be sufficient to accurately diagnose and manage bullous keratopathy, which is where imaging technologies and biomarkers come in. Identifying reliable biomarkers holds promise in addressing unmet needs related to early diagnosis, disease monitoring, and personalized treatment strategies for bullous keratopathy.
The need for biomarkers in bullous keratopathy treatment arises from the complexity of this sight-threatening corneal disorder and its treatment challenges. By quantifying changes in corneal epithelium cell (CEC) levels, ophthalmologists can assess the effectiveness of therapeutic interventions and can adjust bullous keratopathy treatment strategies accordingly.
Diagnostic tools also play an important role in identifying the disease; a study monitoring endothelial disruption, and iridocorneal adhesion in an experimental mouse model suggests an anterior segment optical coherence tomography (AS-OCT) may serve as an adjunct to clinical diagnosis in cases of bullous keratopathy. AS-OCT is a non-contact method of in vivo ocular imaging technology that provides insights into corneal epithelium size and thickness that is believed to be thinned out in patients suffering from bullous keratopathy.
Early diagnosis is essential to address the underlying causes before bullous keratopathy develops to advanced stages, resulting in permanent vision loss in patients. Advanced imaging technologies and biomarkers will revolutionize the diagnosis, management, and research of corneal epithelium diseases, offering new hope for patients and guiding clinicians toward targeted personalized therapeutic strategies. Initiatives must be undertaken to educate healthcare professionals and promote awareness among at-risk populations.
Understanding the current bullous keratopathy treatment and management landscape
- No pharmacotherapy is available to treat bullous keratopathy in the United States or in Europe. Hypertonic saline drops and ointment (sodium chloride 5%), antibiotics, anti-inflammatories, anti-glaucoma, lubricating drops, and bandage contact lenses are used to alleviate symptoms. However, these medicinal therapies are only beneficial in the early stages of bullous keratopathy and do not provide any long-term solution.
- Corneal transplantation remains the mainstay of bullous keratopathy treatment for most vision loss, endothelial cell dysfunctions, or irreversible endothelial decompensation. Endothelial transplantation, either by Descemet membrane endothelial keratoplasty (DMEK) or by Descemet stripping (automated) endothelial keratoplasty (DS(A)EK), is a surgical approach that replaces diseased Descemet membrane and endothelium with tissue from a healthy donor eye. When this is not possible, a penetrating keratoplasty that involves replacing the entire cornea with a donor cornea is considered. Keratoprosthesis, or artificial corneal transplant, is another clinical approach to corneal replacement. This is often reserved for high-risk individuals who have experienced multiple graft failures.
- Keratoprosthesis is associated with postoperative complications such as inflammation, corneal melt and interface issues, and the development of glaucoma, and thus requires rigorous, life-long post-operative care. Additionally, depending on the severity of the bullous keratopathy and co-existing corneal edema, different treatment methods such as conjunctival flaps, anterior stromal puncture, amniotic membrane transplantation, and phototherapeutic keratectomy may be used alone or in combination.
- All these surgical therapies are complex and demand highly trained professionals and well-developed surgical infrastructure. Even though only donor tissue that meets stringent criteria is used, immunological rejection may hamper outcomes. Additionally, access to these therapies has been restricted by high treatment costs, lack of donor tissue, and an increasing aging population, resulting in increased patient needs.
Cell therapy for bullous keratopathy treatment: A promising frontier
Years of research into pharmacological approaches that promote endogenous corneal endothelial regeneration have contributed to the evolution of novel corneal cell therapy that is not only painless but also overcomes the limited regenerative capacity of HCECs for various transplants.
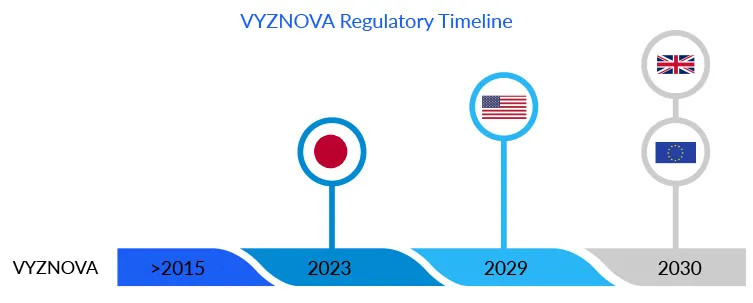
Due to the lack of donor corneas and the related technological constraints, much effort has been directed toward cell therapy as an alternative to corneal surgery. VYZNOVA, which was recently approved in Japan, is one such example. According to DelveInsight’s estimates, the bullous keratopathy drug is likely to cross USD 5 million in Japan by 2030.The approval of this first allogenic cell therapy marked a significant advancement in the bullous keratopathy treatment. Based on a unique concept that allows healthy cells from a donor cornea to cultivate in a multistep, proprietary, and patented manner. Unlike stem cells, these fully differentiated corneal endothelial cells regenerate outside the body, using cultured cells from a healthy single donor, to create treatments for more than 100 recipient eyes. The endothelial cells are injected intracamerally into the eye, repopulating to form a healthy monolayer, and drain fluid from the cornea, reducing corneal edema. Usually, HCECs degrade or damage with age, eye diseases, or surgical trauma, as an individual has a finite number of HCECs thus, replicating these cells in the laboratory has proven to be a game changer in the bullous keratopathy treatment regime for corneal endothelium diseases.
VYZNOVA findings are extraordinary; significant anatomic and functional improvements with full vision recovery were found in highly edematous corneas with bullous keratopathy eyes.
MD, Cincinnati Eye Institute, US
This new cell therapy for bullous keratopathy treatment improves existing therapies (penetrating and endothelial keratoplasties) by simplifying treatment and eliminating potential problems such as graft separation, transplant rejection, dislocation, uneven astigmatism, and infection. Moreover, the cell therapy procedure is minimally invasive and can be performed relatively rapidly, with a recovery period of approximately 2–3 h for patients.
After cell-therapy treatment, the corneal edema is reduced to normal levels, and their visual acuity is significantly improved which is remarkable.
Hudson Medical Center, Hudson, US
Aurion Biotechnologies now expects to gain approval for the drug in the US and EU as well; however, the company must conduct trials in the respective regions.
Cell therapies, acellular graft alternatives, and pharmacological and genetic regulation of the corneal endothelium are among the additional techniques identified for research and development for corneal endothelial regeneration. In addition, procedures such as acellular corneal endothelial graft equivalents and specialized medications can provide a treatment alternative for specific disease conditions without using donor tissue or cells.
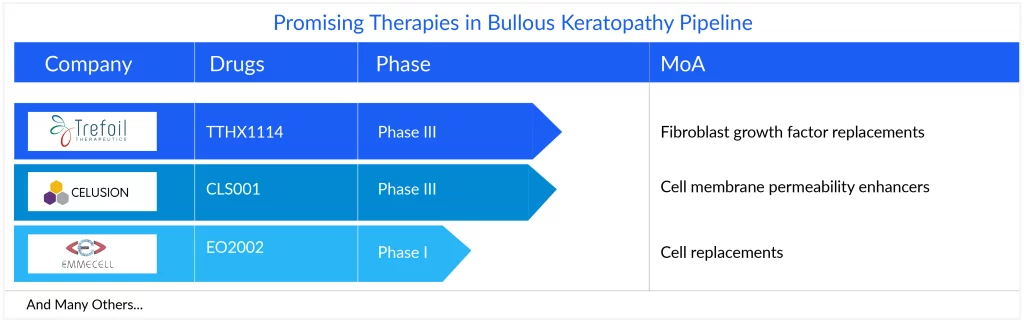
The current emerging pipeline of bullous keratopathy treatment is scarce, with no late-stage product. However, assets in the early stage, including TTHX1114 (NM141) of Trefoil Therapeutics, Emmecell’s EO2002, and Cellusion’s CLS001, offer hope for the future. A few assets, namely CLS004 and DWR-2206/AE101, are in the preclinical stages of development for bullous keratopathy treatment.
TTHX1114 (NM141), a novel engineered FGF1 (eFGF1) compound is being developed as an intracameral injectable formulation, designed to generate the endothelial cell layer and reduce or eliminate the symptoms associated with the condition that leads to vision loss. The drug has completed Phase I/II trials in patients with pseudophakic bullous keratopathy. While Emmecell’s EO2002, currently under Phase I, is a cell-based therapy that utilizes a magnetic cell delivery nanoparticle platform to enhance the delivery, retention, and integration of cell therapies at the target tissue. Cellusion’s CLS001 is a novel regenerative medicine product for bullous keratopathy treatment which is based on the combination of CECSi cells made from iPS cells with excellent proliferative properties that can cure bullous keratopathy. The therapy is also under Phase I trial exploring the safety efficacy of iPS cell-derived corneal endothelial cell substitutes for bullous keratopathy after corneal transplantation.
Delivering cells to the inner layer of the human cornea is another challenge; several approaches, ranging from simple injection to artificial corneal scaffolding, have been researched. Despite unresolved problems, corneal endothelial cell treatments in the clinic represent the future of corneal endotheliopathies treatment and may be viable alternatives.
FAQs
Bullous keratopathy is a pathological condition in which small vesicles, or bullae, formation occurs in the cornea due to endothelial dysfunction. Initially, there is endothelial trauma, followed by progressive stromal and epithelial edema. The epithelial edema results in the formation of bullae, hence the name bullous keratopathy.
Individuals with bullous keratopathy may experience a range of symptoms. Early stages of the condition may present with mild discomfort, eye redness, and blurred vision. As the disease progresses, patients may develop severe eye pain, sensitivity to light, and decreased vision.
Diagnosis of bullous keratopathy typically involves a comprehensive eye examination, including visual acuity testing, slit-lamp examination, and evaluation of corneal thickness and clarity. In some cases, additional tests such as corneal topography or specular microscopy are required to assess the severity and extent of corneal damage.
The treatment of bullous keratopathy aims to alleviate symptoms, improve visual function, and manage corneal edema. The treatment choice depends on the severity of the condition and the patient’s circumstances. In the early stages, conservative therapies such as lubricating eye drops, hyperosmotic medicines, anti-glaucoma, steroids, ointments, or bandage contact lenses are used to reduce pain and protect the cornea.

Downloads
Article in PDF