Global Chronic Pain Associated With Painful Diabetic Neuropathy Market Landscape
May 14, 2021
Table of Contents
Neuropathy is the most usual complication that occurs due to diabetes, affecting up to about 50% of the patients. Painful diabetic neuropathy tends to take place in ~25% of the patients suffering from diabetes mellitus. These patients get treated in office settings, and it affects their quality of life. It also occurs in 30% of the patients suffering from diabetes mellitus, and they are often hospitalized.
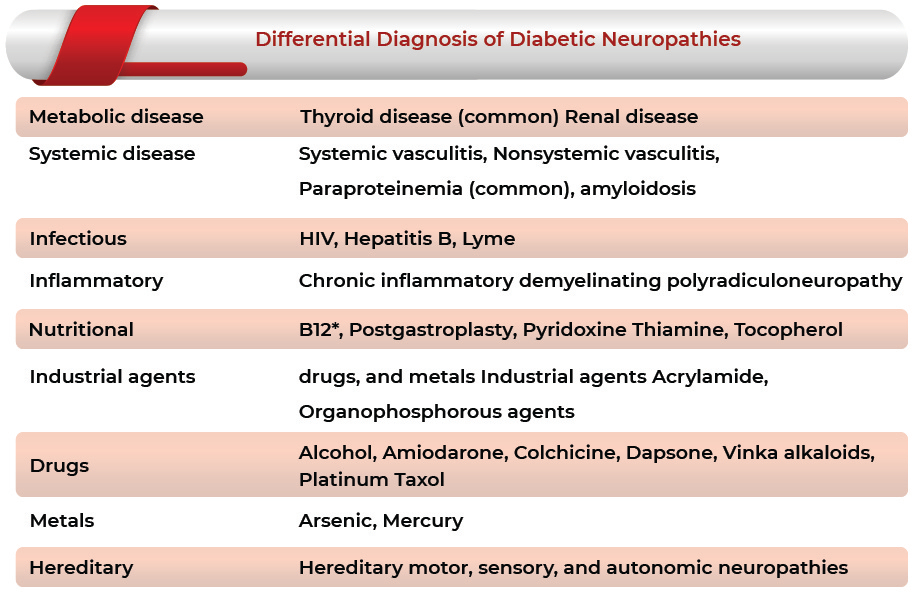
According to the definition given by IASP (International Association for the Study of Pain), Painful Diabetic Neuropathy (PDN) may be defined as a pain arising as a direct consequence of abnormalities in the somatosensory system in people with diabetes. There are many types of neuropathy depending on varying clinical presentations. Peripheral neuropathy can be represented either with painful or painless symptoms. The two most usual forms of diabetic neuropathies associated with pain can be acute sensory neuropathy and chronic sensorimotor neuropathy.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Bioelectronic Medicine: Applications and Future Prospects
- Juvenescence nets USD 100M; Sarepta DMD drug faces rejection
- Promising Data from the First Dedicated Kidney Outcomes Trial with GLP-1 Receptor Agonist, Semagl...
- Notizia
- AbbVie, Sofinnova back; Gilead, Kite ink; Biogen aims filing; Reversing diabetes
Chronic sensory-motor neuropathy is the most common type of diabetic polyneuropathy, which is associated with both symptomatic pain and clinical signs of neuropathy. Painful Diabetic neuropathy typically leads to burning pain, paresthesias, and numbness in a stocking-glove pattern that progresses proximally from the feet and hands. There can be allodynia in the form of cutaneous hypersensitivity that might cause acute distress when in contact with external stimuli, such as clothing.
The symptoms associated with the chronic pain related to painful diabetic neuropathy tend to occur depending on the type and the type of nerves that are affected, which eventually appear with time. One may not notice any defect until a considerable amount of nerve damage has taken place. Chronic diabetic painful neuropathy comprises persistent or episodic pain that might turn worse in the night and tends to improve during walking, localized predominantly in the feet. The pain is often characterized as a deep-seated ache, but there might be superimposed lancinating or burning thermal quality.
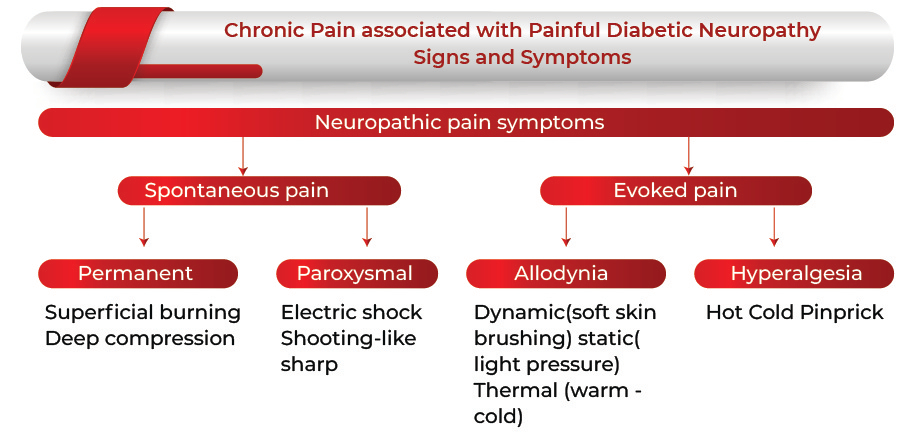
The diagnosis is mostly based on the clinical findings, the type of pain (such as burning discomfort, electric shock-like sensation, aching coldness in the lower limbs) and the time of occurrence (occurs mainly at rest and night), and abnormal sensations (such as tingling or numbness).
Chronic Pain Associated With Painful Diabetic Neuropathy Treatment Options
There are two therapeutic approaches, pathogenic treatments that target the below lying pathophysiological processes to prevent nerve fiber losses and symptomatic therapies that focus on alleviating the painful symptoms of painful diabetic neuropathy to normalize physical and psychological functioning.
Only two medications, namely pregabalin (Lyrica) and duloxetine (Cymbalta), have been approved by the United States Food and Drug Administration (FDA) for the treatment of chronic pain that has been related to diabetic painful neuropathy. The guidelines offered by national organizations such as the American Academy of Neurology had, however, prescribed the use of a broader range of medications. The clinicians consider the patient’s age, health-related quality of goals, physical function and comorbidities, and the most probable side effects from the medication used in the management of pharmacologic therapy. Additionally, medication dosages and the duration of the therapy can be regulated, and the titrations can be performed based on regular patient feedback related to pain relief, improved function, and adverse effects.
There are a vast number of electrotherapies, including transcutaneous electrical nerve stimulation (TENS); pulsed-dose electrical stimulation; peripheral nerve, nerve root, spinal cord, deep brain, and epidural motor cortex stimulation; pulsed (electro-)magnetic fields and static magnetic fields; high-frequency external muscle stimulation; high-tone external muscle stimulation and external muscle stimulation that enable the palliation of chronic pain associated with painful diabetic neuropathy extensively. However, of all these electrotherapies, only TENS is currently recommended as a treatment for painful peripheral diabetic neuropathy by the American Academy of Neurology.
Eligible patients
The pace of population aging around the world is increasing dramatically. The World Health Organization estimates that by 2050, the world’s population aged 60 years and older is expected to total 2 billion, up from 900 million in 2015. In 2015, 125 million people were aged 80 years or older and were estimated to reach 434 million people in this age group worldwide.
Diabetic neuropathy is a global problem affecting about half of the people with diabetes worldwide. The prevalence of PDN rises to approximately 50% when diabetes has been present for more than 25 years and increases with a longer duration of the disease and poor glycemic control. PDN can lead to debilitating painful neuropathy in about 25-30% of patients with diabetes.
According to the American Diabetes Association, 9.4% of the US population had diabetes resulting to give 30.3 million in 2015. Long-standing peripheral neuropathic pain associated with peripheral neuropathy occurs in one of six diabetic subjects. According to International Diabetes Federation estimates, Diabetes has reached epidemic proportions worldwide, suggesting a prevalence of 425 million people worldwide in 2017, rising to 628 million by 2045.
As per DelveInsight’s Chronic Pain associated with Painful Diabetic Neuropathy Market analysis, the total diagnosed prevalent cases of Chronic Pain associated with Painful Diabetic Neuropathy in the 7MM is expected to increase at a CAGR of 0.64% during the study period 2018-30. The largest population for Chronic Pain associated with Painful Diabetic Neuropathy was found in the United States.
Chronic Pain Associated with Painful Diabetic Neuropathy Market Dynamics
The Chronic Pain associated with Painful Diabetic Neuropathy market is estimated to increase due to advancements in healthcare technology, high healthcare expenditure, and available reimbursement policies in developed countries.
Along with this, the growing geriatric population globally, rising prevalence and incidence of diabetes, and technological advancements are factors responsible for the growth of the market of Chronic Pain associated with Painful Diabetic Neuropathy.
However, the availability of alternatives for medical devices like pharmaceuticals options, traditional medicines, and physiotherapy, high cost of devices, and lack of awareness about the treatment devices can deter the market’s growth in the coming years.
According to the DelveInsight estimates, the highest contribution in the market size of Chronic Pain associated with Painful Diabetic Neuropathy was from the pharmacotherapy market 95.2% while the medical device market takes up only 4.8% in 2018, which is expected to increase by 2026 with pharmacotherapy market taking 49.3% of the share and medical device market with 50.57% share in 2026 with the launch of new devices and increased awareness.
Chronic Pain Associated with Painful Diabetic Neuropathy Market Key Companies
The companies operating in the Chronic Pain associated with Painful Diabetic Neuropathy market include Grünenthal, Daiichi Sankyo, Janssen Pharmaceuticals/Grunethal, Pfizer, Eli Lilly and Shionogi, Ono pharmaceuticals, Fremslife S.r.l., Helixmith, Aptinyx, Regenacy Pharmaceuticals, Novaremed, Nevro Corp., Erchonia Corporation, Regenesis Biomedical, Inc., Abbott, and ZyGood LLC., among others.

Developmental Activities
- In July 2020, the US Food and Drug Administration (FDA) approved the first use of a medicated patch made with capsaicin, Qutenza (8% capsaicin) by Grünenthal. This is the first kind of patch to be approved for painful diabetic neuropathy.
- In December 2020, Nevro Corp. submitted a pre-market approval supplement to the US FDA to seek approval of its Senza® System for the treatment of chronic pain associated with painful diabetic neuropathy. The company expects to achieve approval and initiate US launch activities for the Senza System and HF10 therapy to treat chronic pain in PDN patients in the second half of 2021.
Chronic Pain associated with Painful Diabetic Neuropathy Market: Way Ahead
The International Diabetes Federation stated that diabetes is the largest global epidemic of the 21st century, whereas “Diabetes distress” is a term used to describe the hidden emotional and economic burden of diabetes complications. Although at a rate slower than needed, advances are necessary to meet the impending healthcare crisis. Diabetic peripheral neuropathy (PDN) is the most common complication of both type 1 and 2 diabetes and occurs in more than half of affected individuals in both hospital and clinic settings.
The growing geriatric population globally and increasing prevalence of diabetes will act as a market driver for devices used in the treatment of chronic pain-associated PDN. Advances in the current understanding of the clinical presentation and optimal therapeutic management of diabetic neuropathy form the foundation for the ongoing paradigm shift in the preclinical research space.
Various pharmaceutical companies are developing therapies such as VM202 (Helixmith), NYX-2925 (Aptinyx), WST-057 (4% pirenzepine) (WinSanTor, Inc.), Ricolinostat (Regenacy Pharmaceuticals), NRD.E1 (Novaremed Ltd.), Cebranopadol (Grünenthal GmbH), GRC 17356 (Glenmark Pharmaceuticals), and others which are expected to enter the market by 2030 as effective therapies. Among all these therapies, gene therapy, VM202 is expected to hold the highest market share by 2026.
Looking forward to the MedTech market for PDN, two technologies are available in the market, which have been approved over the years by FDA and EMA. Transcutaneous electrical stimulation (TENS) is one low-cost option that patients can administer at home. It has been used as a treatment for diabetic peripheral neuropathy. Many more devices including Erchonia FX-635, Provant® Therapy System, Proclaim™ DRG Neurostimulator, and TransCutaneous Magnetic Stimulation (TCMS) are in pipeline and expected to enter the market by 2030.
The growth of the Chronic Pain associated with the Painful Diabetic Neuropathy market in the coming years will be hindered because of the factors such as lack of awareness among patients, availability of alternative treatments such as physiotherapy and pharmaceuticals.
Downloads
Article in PDF
Recent Articles
- Charting the Growth of the Insulin Pens Market Amidst Rising Demand
- Peloton raises $150M; BiomX raises $32M; Abbott and Novo collaborate; Blaze Bioscience nets $5M
- FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev; Roche and Carmot Ther...
- Nutrition Nexus: Illuminating the Growth Trajectory Path in the Dietary Supplements Market
- CE Mark to Ibex’s Gastric Cancer Detection System; Senseonics’s Eversense E3 Continuous Glucose M...