Endometriosis: The Unseen Burden and the Road Toward Transformative Therapies
Sep 26, 2025
Table of Contents
Endometriosis is a chronic and often debilitating condition where tissue similar to the lining of the uterus, known as the endometrium, grows outside the uterus. This tissue can be found on organs such as the ovaries, fallopian tubes, and the pelvic lining, leading to a range of distressing symptoms. Commonly referred to as “what is endometriosis” in patient searches, this complex disease is characterized by its ability to cause inflammation, scarring, and adhesion formation, which contribute to its hallmark symptoms of endometriosis, including pelvic pain and infertility.
Despite being a common gynecological disorder, the exact cause of endometriosis remains unclear, with theories pointing to genetic, hormonal, and immune system factors. Understanding endometriosis symptoms, such as painful periods, chronic pelvic pain, and pain during intercourse, is crucial for early recognition and management of the condition.
Downloads
Click Here To Get the Article in PDF
Prevalence and Impact on Women’s Health
Endometriosis affects approximately 1 in 10 women of reproductive age globally, translating to over 190 million women worldwide. The prevalence of endometriosis is likely underestimated due to diagnostic challenges, as signs of endometriosis are often mistaken for other conditions or dismissed as normal menstrual discomfort. This disease significantly impacts women’s health, contributing to physical, emotional, and social burdens. Women with endometriosis frequently experience severe endometriosis symptoms that disrupt daily activities, work, and relationships.
The condition is also a leading cause of infertility, affecting up to 30-50% of women with endometriosis who struggle to conceive. The economic burden is substantial, with costs stemming from endometriosis treatments, including endometriosis medication and endometriosis surgery, as well as lost productivity. Increased awareness and research into endometriosis therapies and endometriosis clinical trials are vital to improving diagnosis, treatment, and quality of life for those affected.
Understanding the Endometriosis Epidemiology of Endometriosis
Endometriosis is a highly prevalent condition, affecting approximately 1 in 10 reproductive-aged individuals worldwide, equating to over 190 million women. In the United States alone, diagnosed prevalent cases of endometriosis reached approximately 5 million in 2024, with projections indicating a potential increase by 2034. This high prevalence of endometriosis underscores the urgent need for improved diagnostic tools and endometriosis treatments.
The condition disproportionately affects women in their reproductive years, with the highest number of prevalent cases in the US observed in the 18–29 age group, totaling around 5 million cases in 2024. Despite its widespread occurrence, endometriosis remains underdiagnosed due to the complexity of identifying symptoms of endometriosis and the reliance on invasive procedures like laparoscopy for definitive diagnosis.
Risk Factors and Demographic Trends
Several risk factors contribute to the development of endometriosis, including genetic predisposition, early onset of menstruation, short menstrual cycles, and nulliparity (never having given birth). Demographic trends indicate that endometriosis symptoms are most commonly reported among women aged 18–29, though the condition can persist into later reproductive years. In 2024, US data revealed that diagnosed cases varied by pain severity: approximately 1.2 million cases were mild, 2 million were moderate, and 1.7 million were severe, with all categories expected to rise over the forecast period (2024–2034). These figures highlight the diverse presentation of signs of endometriosis and the need for tailored endometriosis therapies. Racial and socioeconomic disparities also play a role, as access to healthcare and awareness of endometriosis can influence diagnosis rates across populations.
Challenges in Diagnosis and Underreporting
Diagnosing endometriosis remains a significant challenge due to the variability of endometriosis symptoms and their overlap with other gynecological conditions. Chronic pelvic pain, painful menstrual periods, and pain during intercourse are hallmark signs of endometriosis, yet these are often dismissed or misdiagnosed, leading to an average diagnostic delay of 7–10 years. This delay exacerbates the patient burden, as untreated endometriosis can worsen, necessitating more intensive endometriosis treatments, such as endometriosis surgery or long-term endometriosis medication.
Underreporting is a critical issue, as cultural stigmas around menstrual health and limited access to specialized care contribute to undetected cases. Active research and development efforts by pharmaceutical companies, including ongoing endometriosis clinical trials, aim to address these diagnostic challenges and develop non-invasive tools to improve early detection and reduce the burden of this chronic condition.
Pathophysiology of Endometriosis
Understanding the pathophysiology of endometriosis is crucial for elucidating why the disease develops, progresses, and often resists standard therapies in many women. Although widely studied, the condition remains multifactorial, involving a combination of hormonal, immune, and genetic influences that create a permissive environment for ectopic endometrial tissue to thrive.
Mechanisms of Disease Development
Endometriosis is characterized by the growth of endometrial-like tissue outside the uterus, leading to chronic inflammation, adhesion formation, and scarring. The exact mechanisms driving endometriosis remain complex, but key theories include retrograde menstruation, where menstrual blood flows backward into the pelvic cavity, and coelomic metaplasia, where peritoneal cells transform into endometrial-like tissue. Other factors, such as immune dysregulation and genetic predisposition, contribute to the survival and proliferation of ectopic endometrial tissue.
Neoangiogenesis, the formation of new blood vessels, supports the growth of endometriotic implants, while local estrogen production by aromatase enzymes in these implants fuels disease progression. These processes lead to the development of endometriosis symptoms, such as pelvic pain and infertility, making the condition a target for ongoing endometriosis clinical trials exploring novel endometriosis therapies.
Symptoms and Clinical Manifestations
The signs of endometriosis are diverse and often debilitating, with chronic pelvic pain, painful menstrual periods (dysmenorrhea), and pain during intercourse (dyspareunia) being the most common endometriosis symptoms. Other manifestations include heavy menstrual bleeding, fatigue, and gastrointestinal issues, such as bloating or painful bowel movements.
The severity of symptoms varies, with approximately 1,200,000 mild, 1,900,000 moderate, and 1,700,000 severe cases reported in the US in 2024. Infertility affects 30–50% of women with endometriosis, adding to the clinical burden. The unpredictable nature of these symptoms complicates diagnosis and underscores the need for effective endometriosis treatments, including both medical and surgical approaches.
Impact on Quality of Life
Endometriosis imposes a significant burden on physical, emotional, and social well-being. Chronic pelvic pain and other symptoms of endometriosis disrupt daily activities, work, and relationships, leading to reduced productivity and financial strain from medical consultations and treatments. The emotional toll is profound, with many women experiencing anxiety, depression, and isolation due to persistent pain and fertility challenges. The need for repeated interventions, such as endometriosis surgery or long-term endometriosis medication, further exacerbates the economic and psychological impact. The lack of definitive cures and the chronic nature of the disease highlight the importance of advancing endometriosis therapies to improve quality of life.
Current Endometriosis Treatment Landscape
Medical Treatments
Hormonal Therapies
Hormonal therapies are a cornerstone of endometriosis treatment, aiming to suppress endometrial tissue growth and alleviate pain. Gonadotropin-releasing hormone (GnRH) antagonists, such as ORILISSA (elagolix) and RELUMINA (relugolix), work by inhibiting pituitary gonadotropin release, creating a hypoestrogenic state that reduces ovarian hormone levels. ORILISSA, the first FDA-approved oral treatment for moderate-to-severe endometriosis pain, and MYFEMBREE (relugolix, estradiol, and norethindrone acetate), approved in 2022 for up to 24 months of use, are widely used. RYEQO (relugolix combination) received European Commission approval in November 2023 for the treatment of endometriosis symptoms in women who have received prior therapies. Selective progesterone receptor modulators (SPRMs), such as mifepristone, and selective estrogen receptor modulators (SERMs), like bazedoxifene, target specific hormonal pathways, whereas aromatase inhibitors (AIs) reduce local estrogen production in endometriotic implants. However, prolonged AI use may cause ovarian cysts and bone loss, often requiring combination with FSH-suppressing agents.
Pain Management
Non-hormonal pain management strategies, such as nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly used to address endometriosis-related pain. Immunomodulators, like TNF-α blockers (e.g., infliximab), have been explored but show limited efficacy based on small-scale studies. Antiangiogenic agents targeting neoangiogenesis are being investigated in endometriosis clinical trials; however, insufficient clinical evidence currently limits their use. Pain management remains a critical component of endometriosis treatment, particularly for patients with hormone-resistant symptoms.
Surgical Interventions
Laparoscopy and Excision
Laparoscopic surgery is the gold standard for diagnosing and treating endometriosis, involving the excision or ablation of endometriotic implants and adhesions. This approach aims to alleviate pain and improve fertility, though outcomes vary. Excision of endometriomas, while effective for pain relief, may reduce ovarian reserve, posing challenges for fertility preservation. Ongoing research in endometriosis clinical trials aims to refine minimally invasive techniques, enhancing efficacy and reducing complications.
Hysterectomy
Hysterectomy, often considered for severe, hormone-resistant endometriosis, involves the removal of the uterus and sometimes the ovaries, leading to surgical menopause. While effective for pain relief in some cases, it carries risks, including postoperative pain and increased cardiovascular issues due to estrogen loss. Hysterectomy is typically a last resort due to its irreversible impact on fertility and long-term health implications.
Limitations of Current Endometriosis Treatments
Current endometriosis treatments, while effective for some, have significant limitations. Hormonal therapies, such as GnRH antagonists and AIs, may cause side effects like bone loss or ovarian cysts, limiting long-term use. Non-hormonal options, including immunomodulators and antiangiogenic agents, lack robust clinical evidence. Surgical interventions, while beneficial, are invasive, carry risks, and may not provide lasting relief, as endometriosis can recur. The variable efficacy of endometriosis surgery, particularly for assisted reproductive technology outcomes, underscores the need for personalized approaches. The high patient burden and lack of curative options drive the demand for innovative endometriosis drugs and therapies, with pharmaceutical companies actively pursuing solutions through endometriosis clinical trials.
Emerging Endometriosis Therapies and Innovations
Novel Pharmacological Approaches
The pipeline for endometriosis therapies is evolving, with pharmaceutical companies exploring innovative treatments for endometriosis to address unmet needs.
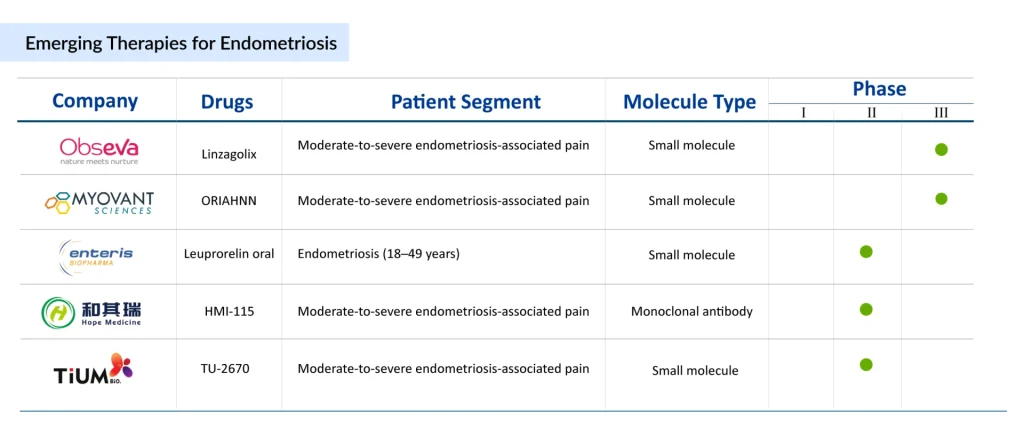
MT-2990, developed by Mitsubishi Tanabe Pharma, is a fully human monoclonal antibody targeting interleukin-33 (IL-33), a cytokine known to promote lesion proliferation and pain in endometriosis. By inhibiting IL-33, MT-2990 aims to reduce endometriosis symptoms and is currently in Phase II clinical trials (NCT03840993).

Another promising candidate, OG-6219, developed by Organon, is a small oral molecule that inhibits estrogen production by targeting the 17β-hydroxysteroid dehydrogenase 1 (HSD17B1) enzyme, acting locally at endometriosis lesions through intracrinology. This approach minimizes systemic estrogen suppression, potentially reducing side effects like bone loss seen in traditional treatments such as GnRH antagonists.
However, OG-6219 failed to meet its primary efficacy endpoint in the Phase II ELENA study (NCT05560646) for moderate-to-severe endometriosis-related pain, leading Organon to discontinue its development in July 2025. These efforts highlight the active pursuit of novel pharmacological solutions in endometriosis clinical trials.
Non-Hormonal Treatment Options
Non-hormonal therapies are gaining attention to address the limitations of hormonal endometriosis treatments, which often cause side effects like bone loss and menopausal symptoms. MT-2990 represents a non-hormonal approach by targeting IL-33, offering a potential alternative for patients with hormone-resistant endometriosis.
Other non-hormonal candidates, such as immunomodulators and antiangiogenic agents, are being explored, though clinical evidence remains limited. For instance, TNF-α blockers, such as infliximab, have shown limited efficacy in small-scale studies, and antiangiogenic agents targeting neoangiogenesis lack sufficient clinical data. The development of non-hormonal endometriosis drugs is critical to providing safer, long-term options for managing endometriosis symptoms, particularly for patients seeking to preserve fertility or avoid hormonal side effects.
Advances in Minimally Invasive Surgery
Advances in minimally invasive surgery are transforming endometriosis treatment, focusing on improving precision and reducing recovery times. Laparoscopic techniques, such as robotic-assisted surgery, enhance the accuracy of endometrioma excision and adhesion removal, minimizing damage to surrounding tissues like the ovarian reserve. These innovations aim to improve outcomes for endometriosis surgery, particularly for patients with severe endometriosis-related pain. Research is also exploring techniques to reduce recurrence rates, as endometriosis can recur after surgery. Ongoing endometriosis clinical trials are evaluating the integration of imaging technologies and intraoperative diagnostics to enhance surgical efficacy, offering hope for better management of signs of endometriosis.
Potential Role of Personalized Medicine
Personalized medicine holds significant promise for endometriosis treatment, leveraging genetic, hormonal, and immune profiles to tailor therapies. Advances in biomarker research aim to identify specific molecular signatures of endometriosis, enabling earlier diagnosis and customized treatment plans.
For example, therapies like MT-2990 could be optimized based on IL-33 expression levels in patients, while intracrinology-based drugs like OG-6219 (prior to its discontinuation) targeted local estrogen production. Personalized approaches could also guide the selection of hormonal versus non-hormonal therapies, optimizing efficacy while minimizing side effects. Endometriosis clinical trials are increasingly incorporating genomic and proteomic data to develop precision medicine strategies, addressing the heterogeneous nature of endometriosis symptoms and treatment responses.
Endometriosis Market Outlook for Endometriosis Treatments
Current Market Size and Growth Trends
The endometriosis market is substantial, with a total market size across the seven major markets (7MM) reaching approximately USD 2.4 billion in 2024, expected to grow by 2034. The United States accounted for the largest share, with a market size of USD 1,300 million in 2024, driven by high prevalence (4,900,000 diagnosed cases) and increasing awareness of endometriosis. Rising diagnosis rates, particularly among the 18–29 age group, and growing demand for effective endometriosis therapies are fueling market expansion. The anticipated increase in diagnosed prevalent cases, projected to rise by 2034, will further drive the need for innovative endometriosis treatments, including both medical and surgical interventions.
Key Players in the Endometriosis Market
Leading companies in the endometriosis market include AbbVie, Neurocrine Biosciences, Bayer, Mitsubishi Tanabe Pharma, Ferring Pharmaceuticals, ObsEva, Kissei Pharmaceuticals, SWK, Enteris BioPharma, Hope Medicine, and Organon. AbbVie’s ORILISSA (elagolix), the first FDA-approved oral treatment for moderate-to-severe endometriosis pain, and MYFEMBREE (relugolix, estradiol, norethindrone acetate) by Myovant Sciences and Pfizer are key marketed therapies. Mitsubishi Tanabe Pharma is advancing MT-2990, while Organon’s OG-6219 was discontinued following its Phase II failure. These companies are actively engaged in endometriosis clinical trials, focusing on novel mechanisms to address unmet needs in endometriosis treatment.
Opportunities and Challenges in Drug Development
The endometriosis market presents significant opportunities due to the high patient burden and demand for effective therapies. Increasing awareness and advocacy are driving investment in endometriosis drugs, with potential for growth in non-hormonal and personalized treatments. However, challenges persist, including high failure rates in clinical trials, as seen with OG-6219, which did not improve pelvic pain compared to placebo. The complexity of endometriosis pathophysiology and variability in symptoms of endometriosis complicate drug development.
Additionally, the high cost of endometriosis surgery and long-term medication, coupled with diagnostic delays, hinders market access. Continued investment in endometriosis clinical trials and innovative approaches, such as MT-2990 and minimally invasive surgical techniques, will be critical to overcoming these challenges and expanding the endometriosis treatment landscape.
Recent Developments in Endometriosis Research
- June 2025: Cicero Diagnostics launched the MyReceptiva endometriosis detection test, a non-invasive blood test to identify patients likely to have endometriosis, facilitating earlier specialist referrals.
- 2025: Heranova Lifesciences introduced HerResolve, a validated laboratory-developed blood test in the US, offering a non-invasive diagnostic option for endometriosis.
- March 2025: Research published in Biomedicines highlighted liquid biopsies as a potential method for detecting biomarkers of endometriosis, with further validation ongoing.
- 2024: A University of Oxford clinical trial explored advanced imaging techniques to improve the detection of superficial and deep abdominal endometriosis lesions, aiming to reduce reliance on invasive laparoscopy.
- In February 2025, Serac Healthcare announced receiving positive feedback from the FDA following its End-of-Phase II Meeting for 99mTc-maraciclatide. This molecular imaging agent is being developed for use with SPECT-CT to visualize and diagnose superficial peritoneal endometriosis (SPE) in women aged 16 and older.
Clinical Trials and Promising Therapies
Endometriosis clinical trials are driving innovation in endometriosis therapies. In March 2025, Michigan State University researchers published findings in iScience, identifying interactions between immune cells and endometriosis lesions, paving the way for non-hormonal treatments targeting immune dysregulation. Mitsubishi Tanabe Pharma’s MT-2990, an interleukin-33 inhibitor, continues to progress in Phase II trials (NCT03840993), showing potential to alleviate endometriosis-related pain without hormonal side effects.
Although Organon’s OG-6219 failed its Phase II trial (NCT05560646) in July 2025 due to insufficient efficacy, other pipeline drugs, such as those from Bayer and Hope Medicine, are under investigation for novel mechanisms. In February 2025, a study published in Frontiers in Endocrinology highlighted the potential role of exosomes as biomarkers and therapeutic delivery systems, offering new avenues for targeted endometriosis treatments. These trials underscore the ongoing effort to develop effective, patient-friendly treatments.
Advocacy and Awareness Initiatives
Advocacy efforts are gaining momentum to address the underdiagnosis and undertreatment of endometriosis. In March 2025, Endometriosis Action Month highlighted global calls for increased funding and awareness, with organizations like Endometriosis UK pushing for reduced diagnostic delays. A September 2025 post on X by @MaryLouMcDonald emphasized the need for specialized endometriosis services in Ireland, reflecting growing public demand for better care. A February 2025 international study published in Drsilina.com linked traumatic experiences to increased endometriosis risk, sparking advocacy for holistic care approaches that address both physical and psychological aspects. These initiatives are driving investment in endometriosis research and improving access to endometriosis medication and surgery, particularly in underserved regions.
Conclusion
The future of endometriosis care lies in overcoming diagnostic delays and developing curative, patient-centered therapies. Non-invasive diagnostics, such as biomarker-based blood tests and advanced imaging, hold promise for earlier detection of signs of endometriosis, reducing the reliance on invasive procedures. Non-hormonal endometriosis drugs, like MT-2990, and personalized medicine approaches tailored to individual genetic and immune profiles could transform treatment outcomes. The endometriosis market is expected to expand by 2034, driven by rising prevalence and investment in innovative therapies. Continued advocacy and funding for endometriosis research will be critical to addressing the physical, emotional, and economic burdens of this disease, ensuring better quality of life for millions of women worldwide.