Long-Awaited Victory: Fabre-Kramer’s Major Depressive Disorder Treatment Drug Gains FDA Approval
Mar 02, 2025
Fabre-Kramer Pharmaceuticals’ major depressive disorder drug, gepirone, faced FDA rejection not once, not twice, but three times since the beginning of the 21st century. Finally, the company can rejoice as the FDA has granted long-awaited approval. On 29 September 2023, the FDA approved Fabre-Kramer Pharmaceuticals’ extended-release gepirone hydrochloride tablets, branded as EXXUA, for major depressive disorder treatment in adults. This privately-held company claims that Exxua is the “first and only approved” antidepressant for major depressive disorder treatment in adults, acting through selective 5HT1a receptor agonism to modulate serotonin activity in the central nervous system. However, the drug’s exact mechanism of action remains to be fully understood.
Major depressive disorder is one of the most prevalent psychiatric disorders. In 2023, the total 12-month diagnosed prevalent cases of MDD in the 7MM was around ~44 million, out of which the highest 12-month prevalent cases were observed in the United States. EU4 and the UK countries accounted for nearly ~22 million cases in 2023, as per DelveInsight.
After examining a sample of more than 5,000 patients, EXXUA’s distinctive approach, which focuses on selectively targeting the serotonin (5HT) 1a receptor, effectively alleviates depressive symptoms while maintaining an acceptable side effect profile. Notably, sexual side effects observed during EXXUA treatment in clinical trials were on par with those experienced by the placebo group, and they did not meet the criteria for inclusion in the Adverse Reaction section of EXXUA’s label. Moreover, EXXUA demonstrated an overall safe profile, with no significant adverse effects on weight, blood pressure, heart rate, or liver function. The most common adverse events reported in clinical trials were mild instances of dizziness and nausea, typically associated with dose adjustments and not necessitating treatment discontinuation.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Mixed Fortunes for Biogen and Sage’ Zuranolone: Approval Under the Cloud of Rejection
- Major Depressive Disorder: Unveiling Market Moves and Commercial Breakthroughs
- Relievant Medsystems Launched Ablation System; Thermo Fisher’s Reproductive Health Assays; EarliT...
- EMA to review Cidara Therapeutics’ Rezafungin; EU Approves Gilead Sciences’ Sunlenca; FDA Approve...
- Will a Robust Pipeline Address the Major Depressive Disorder Treatment Conundrum?
Dr. Anita H. Clayton, MD, the Chair of the Department of Psychiatry & Neurobehavioral Sciences at the University of Virginia School of Medicine, a renowned expert in sexual dysfunction issues in MDD treatment, expressed her enthusiasm for the FDA approval of EXXUA (gepirone ER) in the treatment of major depressive disorder (MDD). According to her, EXXUA, as the first 5-HT1a agonist, demonstrates superior efficacy as MDD monotherapy compared to a placebo, while maintaining similar rates of sexual dysfunction in clinical trials. This represents a significant and valuable new treatment option for patients.
“EXXUA marks a significant breakthrough in addressing Major Depressive Disorder (MDD), a severe and incapacitating condition impacting millions across the globe,” according to Stephen Kramer, M.D., CEO of Fabre-Kramer. “Expanding the spectrum of efficacious treatment choices for healthcare professionals and patients is invaluable. We take pride in introducing this pioneering therapy to individuals seeking a fresh solution for managing their depression and enhancing their quality of life.”
EXXUA’s product label prominently features a boxed warning regarding the potential for suicidal thoughts and behaviors in children and young adults, even though the drug is not officially sanctioned for use in these age groups. However, in their announcement, Fabre-Kramer emphasized that the oral MDD medication does not carry specific safety advisories related to sexual dysfunction and weight gain. The company is gearing up for the launch of Exxua in early 2024.
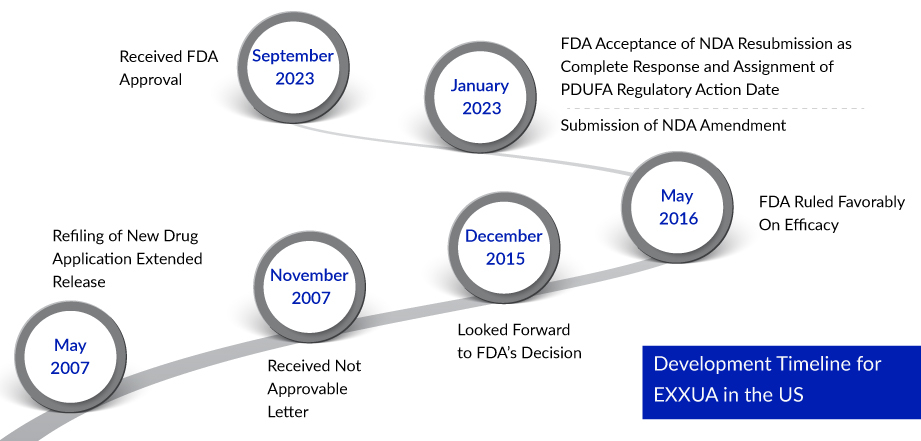
This approval represents the culmination of Fabre-Kramer’s protracted journey through regulatory channels, which began with their initial New Drug Application for gepirone hydrochloride in September 1999 and faced FDA rejection in 2002. Since then, the antidepressant medication has faced numerous regulatory challenges over the years. Notably, in 2015, a meeting of the Psychopharmacologic Drugs Advisory Committee resulted in a 9-4 vote against Fabre-Kramer, citing insufficient evidence from the company to substantiate the drug’s effectiveness. With the successful MDD approval now in hand, Fabre-Kramer is poised to expand the application of EXXUA to address various other psychiatric disorders.
However, several emerging drugs targeting MDD are expected to enter the market in the coming years which will give tough competition to EXXUA. The drugs in the MDD pipeline include Zuranolone (SAGETherapeutics/Biogen), REL-1017 (Relmada Therapeutics), seltorexant (MinervaNeurosciences/Janssen Pharmaceutical), ABV-1504 (BioLite/ABVC BioPharma), and other.
Zuranolone being developed by SAGE Therapeutics and Biogen is a novel, highly potent, and selective, next-generation, an oral, positive allosteric modulator optimized for selectivity to synaptic and extrasynaptic GABAA receptors. The binding sites for NASs are distinct from the recognition sites for GABA, benzodiazepines, and barbiturates. The GABA system is the major inhibitory signaling pathway of the brain and central nervous system (CNS) and contributes significantly to regulating CNS function. SAGE-217 is currently in the pipeline for MDD treatment. It is being evaluated in the LANDSCAPE and NEST clinical trial programs. The two development programs include multiple studies examining the use of zuranolone in several thousand people with a variety of dosing, clinical endpoints, and treatment paradigms. In February 2023, the US FDA accepted the filing of a New Drug Application (NDA) for zuranolone in the treatment of major depressive disorder and postpartum depression. Additionally, the application has been granted priority review and assigned a Prescription Drug User Fee Act (PDUFA) action date in August 2023. Further, trials are ongoing in adolescents and children with MDD.
Seltorexant being developed by Minerva Neurosciences and Janssen Pharmaceutical is a selective orexin-2 receptor antagonist as adjunctive therapy for MDD and for the treatment of insomnia disorder. Seltorexant is the most advanced specific ORX2 molecule in clinical development, with antagonistic activity when binding to its receptor. Seltorexant is currently being developed for two indications, insomnia without associated psychiatric disorders and MDD in patients who have an inadequate response to SSRIs and SNRIs. It is currently being evaluated in Phase III trials.
In May 2024, Johnson & Johnson announced the results of a late-stage trial, revealing that its first-in-class orexin receptor antagonist effectively reduced symptoms of major depressive disorder when used as an adjunctive treatment for patients with insomnia. The company reported that its experimental drug, seltorexant, successfully met all primary and secondary endpoints in a Phase III study involving patients with major depressive disorder and insomnia symptoms.
REL-1017 being developed by Relmada Therapeutics is a new chemical entity (NCE) and novel NMDA receptor (NMDAR) channel blocker that preferentially targets hyperactive channels while maintaining physiological glutamatergic neurotransmission. The drug has also received Fast Track Designation as an immunotherapy for major depressive disorder treatment by the US FDA. In the Phase II trial, REL-1017 demonstrated rapid, robust, and sustained antidepressant effects with statistically significant improvements compared to placebo in tested measures of depression. The Phase II study showed a favorable safety, tolerability, and pharmacokinetics profile of REL-1017. It is currently in late-stage development for the treatment of MDD in adjunctive and monotherapy in Phase III studies. In December 2020, the company reported that the RELIANCE I study did not achieve its primary endpoint, however, with certain improvements in the trials the company is continuing its RELIANCE Phase III Clinical Research Program.
In June 2024, Relmada Therapeutics, Inc. announced the publication of clinical data from its REL-1017 Reliance I Study in the peer-reviewed Journal of Clinical Psychiatry. The article, titled “Efficacy and Safety of Esmethadone (REL-1017) in Patients with Major Depressive Disorder and Inadequate Response to Standard Antidepressants: A Phase 3 Randomized Controlled Trial,” details the findings from the study.
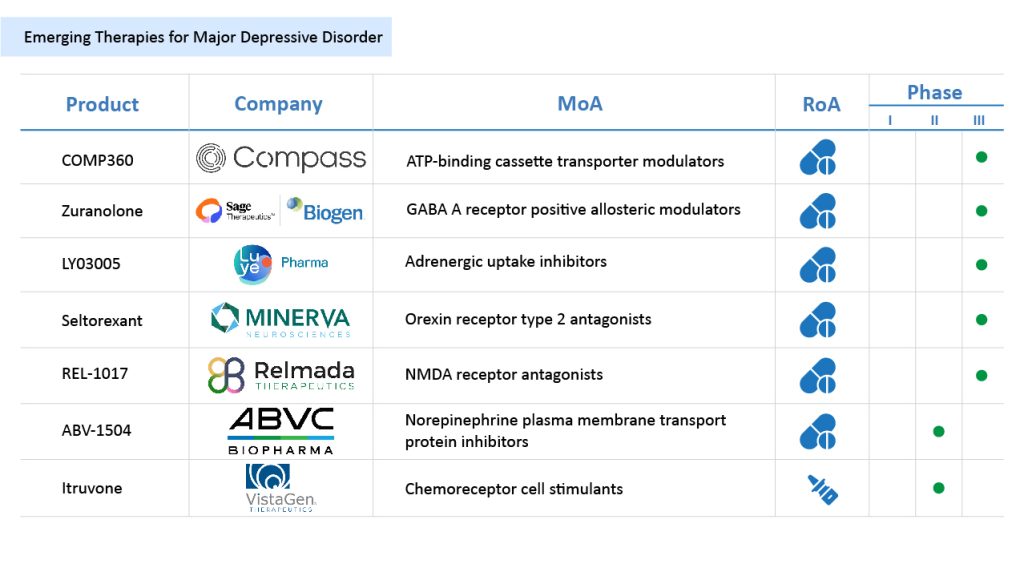
ABV-1504 is being developed by BioLite/ABVC BioPharma, in which PDC-1421 is the active ingredient of the drug, and it is a botanical investigational new drug. Through its subsidiary BioLite, ABVC has completed a Phase II study of the ABV-1504, a botanical-based Norepinephrine Transporter (NET) inhibitor. The study, conducted at Stanford University, found the PDC-1421 capsule to be safe and well-tolerated in effectively treating six enrolled adult patients. Both low and high doses of the PDC1421 capsule passed the required 40% population in ADHD-RS-IV test scores, thus meeting the study’s primary endpoints. In April 2023, PDC-1421 received a US patent certificate, US 11,554,154 B2, for major depressive disorder treatment. The drug has completeda Phase II trial in MDD patients. Further, the company plans to initiate an End of Phase II (EOP II) meeting with the FDA shortly.
With the continuous efforts in research and development and the anticipated launch of these therapies, the major depressive disorder treatment space will heat up in the coming years. As per DelveInsight, the total 7MM major depressive disorder treatment market size was found to be around USD 5.6 billion, which is further expected to increase by 2034 at a CAGR of 4.8%. DelveInsight’s estimates show that the United States accounted for the largest major depressive disorder treatment market size compared to EU4 and the UK, and Japan. It will be interesting to see how these therapies will compete and how EXXUA will maintain its position in the major depressive disorder treatment segment.

Downloads
Article in PDF
Recent Articles
- J&J’s SPRAVATO Achieves Milestone with Monotherapy Approval in Depression Treatment
- Mixed Fortunes for Biogen and Sage’ Zuranolone: Approval Under the Cloud of Rejection
- Relievant Medsystems Launched Ablation System; Thermo Fisher’s Reproductive Health Assays; EarliT...
- Exploring the Impact of AI in Mental Health: How it is Going to Revolutionize Diagnosis and Treat...
- Sage seeks to save depression drug; Regeneron aims clinical trial; J&J sets sights for COVID...



