FDA Approves Omeros’ YARTEMLEA: A Breakthrough for Stem Cell Transplant Patients Facing TA-TMA
Jan 05, 2026
Table of Contents
The FDA has approved YARTEMLEA (narsoplimab-wuug), marking a watershed moment in hematopoietic stem cell transplantation (HSCT) medicine. Omeros Corporation’s therapeutic advance represents the first and only FDA-approved treatment for hematopoietic stem cell transplant-associated thrombotic microangiopathy (TA-TMA). This life-threatening complication has historically lacked effective medical interventions and claimed hundreds of lives annually.
For transplant physicians and their patients, this approval signals a fundamental shift in clinical practice—from managing a fatal disease with supportive measures to wielding a targeted therapy with robust efficacy and survival benefit.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- WASKYRA Wins FDA Approval, Marking a Historic Leap for Rare Disease Gene Therapies
- Zenas BioPharma’s Obexelimab Delivers Positive Phase 3 Results in IgG4-RD; Sanofi Secures US Prio...
- BMS’s LPA1 Antagonist; Alnylam’s KARDIA-1 Phase 2 Study; Day One Biopharma Sought FDA Appro...
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
Understanding TA-TMA: A Rare but Devastating Complication
TA-TMA emerges as a systemic consequence of conditioning regimens, immunosuppression, and the transplant process itself. The condition is characterized by endothelial cell injury, complement pathway activation, and microangiopathic hemolytic anemia, leading to multi-organ dysfunction and failure. The clinical picture is grim: mortality in severe TA-TMA can exceed 90%, with survivors often facing chronic kidney disease and dialysis dependence.
The disease burden is substantial. Approximately 30,000 allogeneic stem cell transplants are performed annually in the United States and Europe. Recent epidemiological studies estimate that TA-TMA develops in up to 56% of allogeneic transplant recipients, translating to roughly 12,000-18,000 patients per year facing this complication. Despite its prevalence and lethality, until now, no FDA-approved therapy existed.
Dr. Miguel-Angel Perales, Chief of Adult Bone Marrow Transplantation at Memorial Sloan Kettering, underscored the clinical vacuum: “Until now, we’ve lacked an effective TA-TMA therapy and relied largely on supportive measures such as modifying calcineurin inhibitors, which can significantly increase the risk of life-threatening graft-versus-host disease.”
YARTEMLEA’s Novel Mechanism: Targeting the Root Cause
YARTEMLEA’s therapeutic approach differs fundamentally from symptomatic management. The drug is a fully human monoclonal antibody that selectively inhibits MASP-2 (mannan-binding lectin-associated serine protease-2), the key effector enzyme of the lectin pathway of the complement system.
In TA-TMA pathophysiology, the lectin pathway becomes aberrantly activated by tissue damage and systemic endothelial injury, triggering a cascade of complement-mediated inflammation, cellular damage, and microvascular thrombosis. By blocking MASP-2, YARTEMLEA interrupts this cascade at its source—while critically, it preserves the classical and alternative complement pathways, maintaining the patient’s adaptive immune response and host defense mechanisms. This selective inhibition provides a therapeutic advantage: treatment addresses the core complement dysregulation driving TA-TMA without compromising infection-fighting capacity.
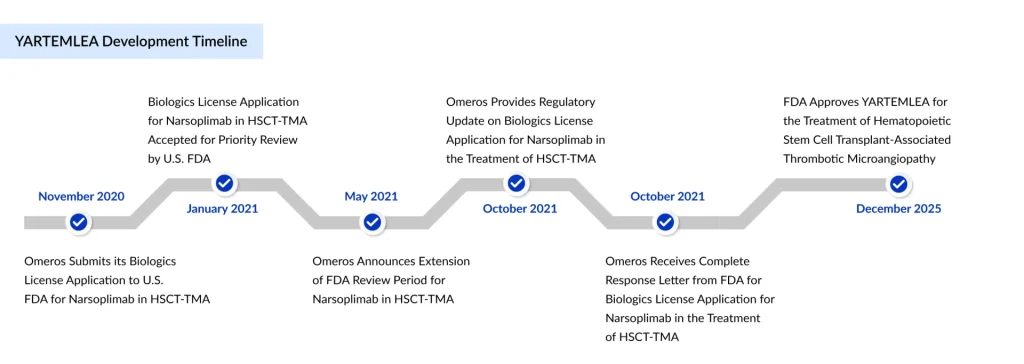
YARTEMLEA’s approval journey was not linear. The FDA issued a complete response letter in 2021, requesting additional efficacy and safety data. Omeros subsequently conducted an expanded access program and provided additional analyses of external registry data, ultimately securing approval on December 24, 2025. This approval establishes several regulatory precedents: the drug received breakthrough therapy and orphan drug designations from the FDA, and the EMA granted orphan drug status with a regulatory decision expected in mid-2026. YARTEMLEA’s FDA approval rested on a robust clinical data package comprising a pivotal open-label trial and an extensive expanded access program.
Pivotal Study Results (N=28 adult patients):
- Complete Response Rate: 61% (defined as improvement in laboratory markers—platelet counts and LDH levels—plus either improved organ function or transfusion independence)
- 100-Day Survival: 73% (95% CI: 52–86%)
- Patient Population: All patients met international criteria for high-risk TA-TMA with poor prognosis
Expanded Access Program Data (N=221 total; 19 evaluable for efficacy):
- Complete Response Rate: 68%
- 100-Day Survival: 74% (95% CI: 48–88%)
- Notable Finding: YARTEMLEA demonstrated efficacy both as first-line therapy and in patients who had previously failed other complement-targeted treatments
The survival improvement becomes even more striking in context: in peer-reviewed publications, the YARTEMLEA treatment was associated with a three- to fourfold reduction in mortality compared to external control cohorts. Among high-risk patients with prior treatment failure, one-year survival reached approximately 50%—a dramatic improvement over historical baseline rates below 20%.
A particularly significant aspect of YARTEMLEA’s indication is its approval for pediatric patients as young as two years old. In the pediatric clinical experience, results have been equally compelling. When used as first-line therapy, YARTEMLEA-treated children achieved approximately 75% one-year survival. Even among pediatric patients refractory to prior complement-inhibiting therapies, survival rates tripled compared to historical benchmarks—rising from below 20% to approximately 60%.
For pediatric hematology-oncology specialists, this approval reshapes the treatment paradigm. Previously, clinicians managing TA-TMA in children relied on off-label complement inhibitors or defibrotide—treatments of uncertain efficacy and variable safety profiles. YARTEMLEA now offers an FDA-approved, targeted alternative with documented pediatric safety and efficacy.
In the context of high-risk TA-TMA patients, many with multi-organ dysfunction and poor baseline prognosis, YARTEMLEA demonstrated a favorable safety profile. Notably:
- No Boxed Warning and no mandatory REMS (Risk Evaluation and Mitigation Strategy)
- No pre-treatment vaccination required, simplifying clinical management
- Most common adverse reactions (≥20%): viral infections, sepsis, hemorrhage, diarrhea, vomiting, nausea, neutropenia, pyrexia, fatigue, and hypokalemia—attributable primarily to the underlying disease severity and transplant-related complications
Serious infections occurred in 36% of treated patients, consistent with expectations in immunocompromised HSCT populations. In the expanded access program, no new or unexpected safety signals emerged, providing reassurance regarding long-term safety.
Clinicians are advised to monitor patients with active infections closely for worsening disease, and management should include prompt treatment of any infection. However, the absence of a Boxed Warning and REMS reflects the favorable benefit-risk calculus compared to prior off-label alternatives.
Competitive Landscape and First-Mover Advantage
YARTEMLEA’s approval establishes Omeros as the sole approved player in an entirely new therapeutic category: MASP-2 inhibition for TA-TMA. The company’s first-mover advantage is substantial. Historically, off-label use of complement inhibitors such as eculizumab (SOLIRIS, targeting C5) or alternative pathway inhibitors provided the only medical interventions, and their efficacy in TA-TMA was suboptimal and variable.
YARTEMLEA’s targeted lectin pathway inhibition, combined with robust clinical data and now regulatory approval, positions it as the new standard of care for TA-TMA. No direct head-to-head trials comparing YARTEMLEA to eculizumab have been conducted. Still, the compelling real-world survival data and mechanism-of-action advantage provide a strong clinical rationale for preferential use in TA-TMA.
Key Takeaway: A New Era in Transplant Care
The FDA approval of YARTEMLEA represents far more than a regulatory milestone—it marks a clinical paradigm shift. For the estimated 12,000–18,000 patients annually who develop TA-TMA after stem cell transplantation, YARTEMLEA offers realistic hope where historically there was only resignation to high mortality. For transplant physicians, YARTEMLEA provides an evidence-based therapeutic tool that directly addresses the pathophysiologic drivers of TA-TMA rather than merely managing symptoms.
YARTEMLEA’s approval validates Omeros’ scientific focus on complement-mediated diseases. The company’s pipeline includes multiple complement-targeted programs, positioning it as a leader in this therapeutic area. Future studies may explore YARTEMLEA’s potential in other complement-driven conditions beyond TA-TMA, though the current indication remains the approved indication.
With a January 2026 U.S. launch, dedicated reimbursement infrastructure, and comprehensive patient support programs, Omeros has positioned YARTEMLEA for rapid clinical uptake and broad access. As the first and only approved therapy for TA-TMA, YARTEMLEA sets a new standard of care—transforming outcomes for adults, children, and families facing this devastating post-transplant complication.

Downloads
Article in PDF
Recent Articles
- Zenas BioPharma’s Obexelimab Delivers Positive Phase 3 Results in IgG4-RD; Sanofi Secures US Prio...
- WASKYRA Wins FDA Approval, Marking a Historic Leap for Rare Disease Gene Therapies
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
- BMS’s LPA1 Antagonist; Alnylam’s KARDIA-1 Phase 2 Study; Day One Biopharma Sought FDA Appro...



