Regeneron Presents Positive Pivotal Data on Linvoseltamab for Relapsed/Refractory Multiple Myeloma at AACR Annual Meeting 2024
Apr 08, 2024
Regeneron Pharmaceuticals unveiled promising results from the Phase I/II LINKER-MM1 trial of linvoseltamab in patients grappling with relapsed/refractory (R/R) multiple myeloma (MM) during the oral plenary session at the American Association for Cancer Research (AACR) Annual Meeting 2024 in San Diego.
Linvoseltamab, an investigational bispecific antibody, demonstrates its potential by bridging B-cell maturation antigen (BCMA) on multiple myeloma cells with CD3-expressing T cells, thereby triggering T-cell activation and subsequent cancer-cell elimination.
Key Findings:
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Rare Cancer Market: A Global Crusade on what is a ‘less common cancer’?
- Merck’s ADC SKB264 Shows Promising Results in Advanced Gastric Cancer: Phase 2 Data Reveals Poten...
- TALVEY’s Versatility Shines: Key Insights from MonumenTAL-2 Study Illuminate Effective Stra...
- Cilta-Cel’s Prolonged Impact: Deep Responses and Safety Insights from Earlier Lines of Ther...
- Wave Life Sciences stocks implode; Amarin’s VASCEPA approval; GSK seeks approval
- Among 117 patients with relapsed/refractory multiple myeloma, linvoseltamab demonstrated a robust 71% objective response rate (ORR), with 46% achieving a complete response (CR) or better, and 62% achieving a very good partial response (VGPR) or better.
- Rapid onset of response was observed, with a median time to response of 1 month and a median time to achieving VGPR or better of 3 months.
- Median duration of response (DoR), progression-free survival (PFS), and overall survival (OS) were not reached. At 12 months, the estimated probabilities of maintaining response, progression-free survival, and survival were 78%, 69%, and 75%, respectively.
- Notably, 93% of patients who achieved CR or better and underwent minimal residual disease (MRD) evaluation were MRD negative at a sensitivity of 10-5.
Linvoseltamab’s journey towards regulatory approval is expedited, having been granted Fast Track Designation and accepted for Priority Review by the FDA for the treatment of R/R MM, with a target action date of August 22, 2024. Concurrently, the European Medicines Agency (EMA) is reviewing its application. It is crucial to note that while linvoseltamab is currently under clinical development, its safety and efficacy await comprehensive evaluation by regulatory authorities.
The Phase III confirmatory trial for linvoseltamab in patients with R/R MM (LINKER-MM3) is currently underway, poised to further delineate its therapeutic potential.
The landscape of bispecific antibodies in the multiple myeloma area is rapidly evolving, with numerous candidates under research. Janssen’s approval of TECVAYLI (teclistamab) in 2022 marks a significant milestone, positioning them strongly in this space. TECVAYLI, a bispecific T-cell engager, targets recurrent or refractory multiple myeloma after extensive prior treatments. Janssen, already a major player with DARZALEX/DARZALEX FASPRO, strengthens its presence with TECVAYLI and TALVEY (talquetamab) approvals. Competitors like Linvoseltamab will face formidable challenges from Janssen’s offerings as well as Pfizer’s ELREXFIO (elranatamab).
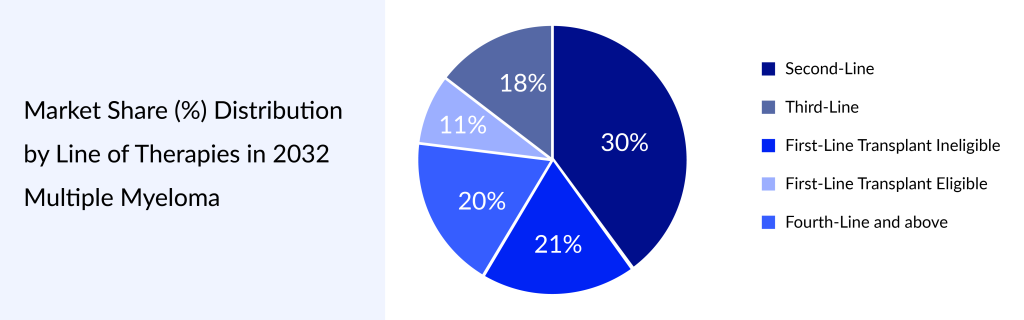
For more information, refer to Delveinsight’s reports:
Multiple Myeloma Market Insight, Epidemiology And Market Forecast – 2034
Downloads
Article in PDF
Recent Articles
- Bristol Mayer Squibb — The Undisputed Leader in the Molecular Glue Arena
- Notizia
- Amgen’s Olpasiran Candidate; GSK’s Novel Antibiotic for Urinary Tract Infections; AstraZeneca and...
- Inoculating Hope: The Landscape of Cancer Vaccination
- BeiGene’s BRUKINSA Gets FDA Accelerated Approval; GSK’s Positive Results in DREAMM-8 ...



