Spinal Muscular Atrophy Emerging therapies
Aug 11, 2016
Spinal Muscular Atrophy (SMA) is a rare genetic and neuromuscular condition which affects mainly children. It has several distinct types such as type 1 (Werdnig-Hoffmann disease), type 2 (Dubowitz disease), type 3 (Kugelberg-Welander disease) and type 4 (Adult onset). SMN1 (Survival Motor Neuron 1) is the primary gene whose mutation causes SMA. The condition mainly affect crawling and walking ability, arm, hand, head and neck movement, breathing and swallowing.
The estimated frequency of spinal muscular atrophy is 1 in 4,000 to 1 in 7,000 people. Approximately one out of every 40 individuals in the United States is a carrier of the gene responsible for spinal muscular atrophy.
We are reaching close with a great hope
As of August 2016, no drugs for SMA have been approved. Nusinersen is leading the race among all the therapies that are currently being evaluated in different stages of clinical development. We have got very good news about this promising drug. In August 2016, biotechnology firms Biogen and Ionis Pharmaceuticals reported that they had decided to cut short a phase-3 clinical trial for the investigational drug Nusinersen, because the drug already “has met the primary endpoint pre-specified for interim analysis” of results. Based on the results of the pre-specified interim analysis, the ENDEAR study will be stopped and participants will be able to transition into the SHINE open-label study in which all patients receive nusinersen. Biogen has option to develop and commercialize Nusinersen globally and paid Ionis a $75 million license fee. Biogen will initiate regulatory filings globally in the coming months.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Gene Therapy in Rare Disorders: Acceptance in Europe Faces Challenges
- Spinal Muscular Atrophy Market is expected to augment at a CAGR of 10.42%
- FDA Expands GSK’s Jemperli Approval; Biogen to Acquire Reata Pharma; Enhertu Shows Survival Boost...
- The era of Gene therapy, and the Billion Dollar tag
- 7 Emerging Spinal Muscular Atrophy Therapies Offering Hope for Patients
Pipeline Scenario
Researchers are putting sincere efforts to develop a reliable treatment therapy for this devastating condition. As a result, we currently have a decent number of pipeline products in various stages of development. Of the total 26 products in the pipeline, 22 are active whose safety, efficacy and tolerability are being studied for spinal muscular atrophy. There are 3 dormant products and 1 product has been discontinued due to termination of collaboration agreement.
A recent evaluation of clinical development revealed that there are many companies competing in this domain, such as Hoffmann-La Roche, Isis Pharmaceuticals, AveXis, Inc., Cytokinetics, Inc., and Novartis AG. Nusinersen by Isis Pharmaceuticals is currently demonstrated encouraging interim Phase III results.
Also, there are three drugs that currently in Phase 2 and five in Phase 1. These include Olesoxime (TRO19622), LMI070, RO6885247, CK-2127107, and RG3039.
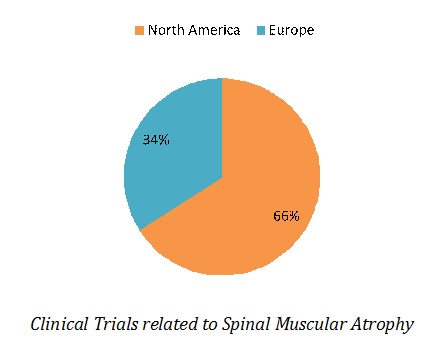
Most of clinical studies are underway in North America (35 clinical trials), followed by Europe (14 clinical trials). In North America, 27 clinical studies are being conducted in United States and 8 in Canada.
.
As discussed above, based on encouraging interim Phase 3 results, Biogen is planning to apply for regulatory approval in the US this year. If approved, this would be the first FDA-approved drug to treat SMA
Insight by:
Mohammad Rizwan
Associate Analyst
DelveInsight Business Research, LLP
Downloads
Article in PDF
Recent Articles
- Biogen’s SPINRAZA Phase II/III Trial Results; Travere’s FILSPARI FDA Approval; GSK’s ...
- How Spinal Muscular Atrophy Therapies transform market?
- FDA’s Ok to Roche’s Oral SMA Therapy; Roche’s Etrolizumab Mixed Results in Ulce...
- Meet the World’s most expensive drug: Zolgensma
- Transforming Pompe Disease Care: Latest Innovations and Strategic Advances in Treatment