Alzheimer’s Disease Diagnostic
Nov 27, 2025
Bracco Receives FDA Clearance for Additional Clinical Use of Max 3™ Syringeless MR Injector; RapidAI Broadens Clinical AI Portfolio with Five Newly Granted FDA Clearances; Ceribell Secures FDA 510(k) Clearance for Clarity® Algorithm in Neonatal Care; BD Introduces Surgiphor™ Irrigation System Across Europe to Enhance Surgical Patient Protection; Neurophet and The Florey Announce Strengthened Collaboration to Advance Alzheimer’s Diagnostic Solutions; VSI® Performs First-Ever Robotic Minimally Invasive Bertolotti’s Resection; BioWave® Demonstrates Safe and Effective Home-Based and War Injury Pain Relief in Latest Clinical Data
FDA Approved Expanded Indication for Max 3™ Syringeless MR Injector from Bracco On November 25, 2025, Bracco Diagnostics Inc., the U.S. subsidiary of Bracco Imaging S.p.A., announced that the U.S. Food and Drug Administration (FDA) expanded the indication for its Max 3™ Rapid Exchange and Syringeless Injector fo...
Read More...
Aug 21, 2025
FDA Grants 510(k) Clearance to Nerveblox AI; Labcorp Introduces the First FDA-Cleared Alzheimer’s Blood Test; Calidar Introduces First-of-its-Kind 4D Mammography System; ONWARD Medical to Initiate Empower BP Pivotal Study Following FDA IDE Approval; Tenon® Medical Launches Catamaran® SE SI Joint Fusion System Nationwide; Rivanna Secures $800,000 Virginia Catalyst Grant Plus Matching Funds to Advance AI-Driven Ultrasound Innovation
Nerveblox, the Ultrasound AI for Regional Anesthesia Received FDA Clearance On August 18, 2025, Nerveblox, an AI-powered software developed by SmartAlpha to assist physicians in performing ultrasound-guided regional anesthesia, received U.S. Food and Drug Administration (FDA) 510(k) clearance. This milesto...
Read More...
Jul 31, 2025
Philips Secures FDA Nod for Image-Guided Platform in Prostate Cancer Treatment; Roche Gains CE Mark for Alzheimer’s Screening Blood Test; CorTec Completes First Human Implantation of Its Brain-Computer Interface in Neurotech Breakthrough; Natera Initiates ABCSG 61 Trial to Assess Signatera™ in Early-Stage Breast Cancer; GE HealthCare Introduces Advanced Digital X-ray System to Enhance Imaging Accessibility in High-Volume Settings; Stryker-Owned Inari Medical Launches InThrill® System for AV-Access and Small Vessel Thrombus Treatment
Philips Secured FDA 510(k) Clearance for Image-Guided Navigation Technology, Advancing Minimally Invasive Prostate Cancer Treatment On July 23, 2025, Philips, a global leader in health technology, announced a significant milestone in prostate cancer care with the U.S. FDA 510(k) clearance of the latest ver...
Read More...
Jun 26, 2025
GE HealthCare Receives FDA Approval for Expanded Vizamyl PET Imaging Indications in Alzheimer’s Disease Care; FDA Grants Approval to InspireMD’s CGuard® Prime for Carotid Artery Disease; AngioDynamics Launches RECOVER-AV Trial, Marking Milestone in AlphaVac F18⁸⁵ Development for PE Treatment; Tivic Health Concludes Study Optimizing Non-Invasive Vagus Nerve Stimulation Device for Personalized Therapy; Johnson & Johnson Introduces VOLT™ Plating System and ACUVUE OASYS MAX 1-Day MULTIFOCAL for ASTIGMATISM in the U.S.
GE HealthCare Announced that the FDA Approved Expanded Indications for Vizamyl Pet Imaging Agent for Beta Amyloid Detection, Enabling More Precise Care for Alzheimer’s Patients On June 24, 2025, GE HealthCare announced that the U.S. Food and Drug Administration (FDA) approved an updated label for its PET i...
Read More...
May 22, 2025
FDA Clears Fujirebio’s Lumipulse® G Plasma Test for Detecting Alzheimer’s-Linked Amyloid Pathology; Stryker Gains FDA Clearance for OptaBlate® BVN System to Treat Chronic Low Back Pain; Abbott Unveils Clinical Evidence Linking Libre® CGM to Reduced Cardiovascular Events in Diabetes; GE HealthCare Launches AI-Based CleaRecon DL for Enhanced 3D Reconstruction in Interventional Suites; Philips Advances Cardiac Care with Launch of RADIQAL Clinical Study and Real-Time 3D Intracardiac Imaging Catheter in Europe
Fujirebio Received FDA Clearance for Innovative Lumipulse® G Plasma Biomarker Test, Marking Major Advancement in Identifying Amyloid Pathology Linked to Alzheimer’s Disease On May 16, 2025, Fujirebio announced that the U.S. Food and Drug Administration (FDA) granted 510(k) clearance for its Lumipulse® G pT...
Read More...
Aug 10, 2023
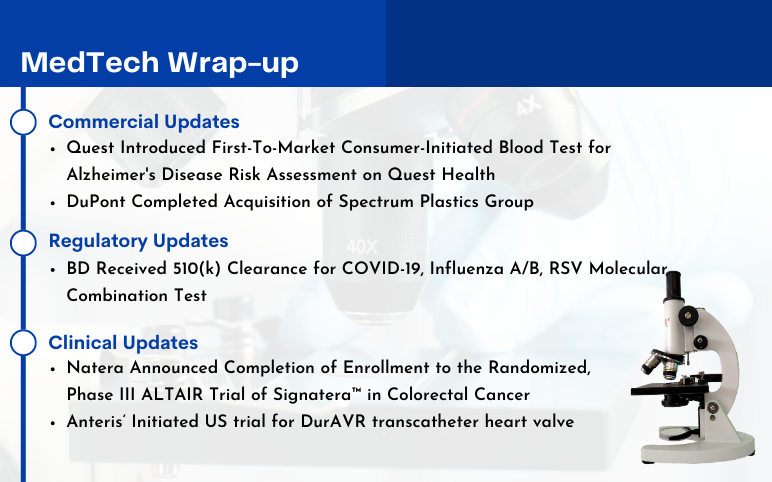
Quest Introduced Consumer-Initiated Blood Test for Alzheimer’s Disease Risk Assessment; DuPont Acquires Spectrum Plastics; BD Received 510(k) Clearance for Molecular Combination Test; Natera Updates on Phase III ALTAIR Trial of Signatera; Anteris’ DurAVR transcatheter heart valve Trial
Quest Introduced First-To-Market Consumer-Initiated Blood Test for Alzheimer's Disease Risk Assessment on Questhealth Website On July 31, 2023, Quest Diagnostics, a leader in diagnostic information services, announced the introduction of the AD-Detect™ Test for Alzheimer's Disease on questhealth.com – the first ...
Read More...
Jun 29, 2023
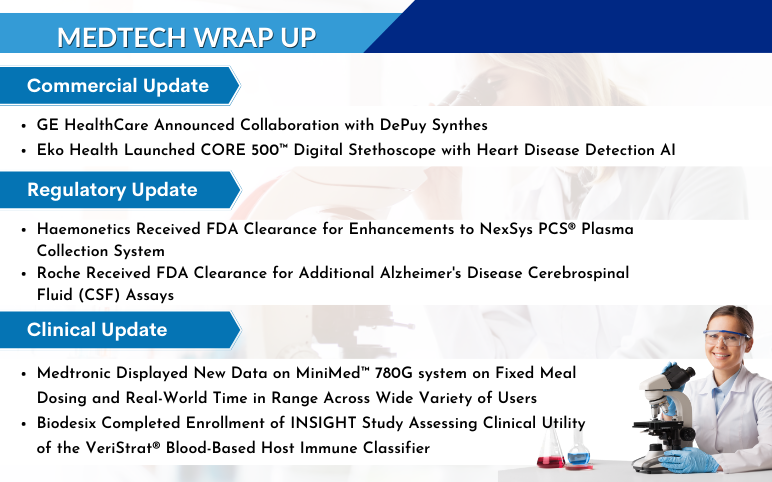
GE HealthCare, DePuy Synthes Announced Collaboration; Eko Health Launched CORE 500 Digital Stethoscope; Haemonetics’s NexSys PCS Plasma Collection System; Roche’s Alzheimer’s Disease Cerebrospinal Fluid (CSF) Assays; Medtronic’s MiniMed 780G system; Biodesix’s VeriStrat Blood-Based Host Immune Classifier
GE HealthCare Announced Collaboration with DePuy Synthes to Bring Advanced 3D Precision Imaging Innovation to Spine Practices in the United States On June 21, 2023, GE HealthCare entered into a distribution agreement with DePuy Synthes, the Orthopaedics division of Johnson & Johnson, to bring GE HealthCare’s...
Read More...




-Agonist.png)

