Cancer diagnostics
Aug 07, 2025
SetPoint Medical Earns FDA Nod for First-of-Its-Kind Neuroimmune Modulation Therapy in RA; Body Vision Medical Secures Singapore Pre‑Market Approval for LungVision; BioLab Holdings Initiates Landmark Clinical Trial for Placental Membrane Therapies; Concept Medical Launches MAGICAL-SV IDE Trial; Frenova Enters Strategic Partnership to Push Boundaries in Kidney Precision Medicine; Demetra Acquires OrthoFundamentals and Establishes Global Spine Division
SetPoint Medical Received FDA Approval for Novel Neuroimmune Modulation Therapy for Rheumatoid Arthritis On July 31, 2025, SetPoint Medical, a company focused on developing therapies for individuals with chronic autoimmune diseases, announced that the U.S. Food and Drug Administration (FDA) approved its Se...
Read More...
Jun 05, 2025
Guardant Health’s Shield Test Earns FDA Breakthrough Device Status; Reflow Medical Gains FDA De Novo Clearance for Spur Peripheral Stent System; FastWave Medical Achieves First-in-Human Use of Coronary Laser IVL System; BD Initiates Patient Registry to Assess Rotarex™ System in PAD Treatment; Johnson & Johnson Unveiled the KINCISE™ 2 System for Use in Knee and Hip Revision Surgeries; Cardinal Health Launches Single-Device Solution for Real-Time Tracking of Three Vital Signs
Guardant Health Secured FDA Breakthrough Device Designation for Its Shield Multi-Cancer Detection Test On June 03, 2025, Guardant Health, Inc., a precision oncology company, reported that the U.S. Food and Drug Administration (FDA) granted breakthrough device designation to its Shield multi-cancer detectio...
Read More...
May 29, 2025
Olympus Gains FDA Clearance for EDOF™ Imaging Endoscopes; FDA Approves Abbott’s Tendyne™ Device for Minimally Invasive Mitral Valve Replacement; New Study Validates Exact Sciences’ Oncodetect™ for Enhanced MRD Detection in Stage II-IV Colorectal Cancer; Sensome Completes First-in-Human Trial Enrollment for Lung Cancer Detection System; J&J MedTech Introduces SOUNDSTAR CRYSTAL™ to US Market with Breakthrough 2D Imaging Quality; Terumo Launches ROADSAVER™ Carotid Stent System to Improve Outcomes in Carotid Artery Stenosis
Olympus Secured FDA Clearance for Cutting-Edge EDOF™ Imaging Endoscopes, Redefining Visibility with Sharper, Blur-Free Views On May 27, 2025, Olympus Corporation, a global leader in medical technology and endoscopic imaging, announced that it received FDA 510(k) clearance for its next-generation EZ1500 series en...
Read More...
Nov 27, 2024
AI in Cancer Diagnostics: Paving the Way for Early Detection and Precision Treatment
Artificial Intelligence (AI) is revolutionizing numerous fields, and healthcare, particularly cancer diagnostics, is no exception. With the promise of faster, more accurate diagnoses, AI is transforming how clinicians detect, diagnose, and treat cancer. By leveraging vast amounts of medical data, advanced algorithm...
Read More...
Nov 14, 2024
FDA Grants Approval to Caris Life Sciences for MI Cancer Seek; Johnson & Johnson MedTech Secures FDA IDE Approval for OTTAVA; Organogenesis Shares Positive Interim Data from Second Phase 3 Study of ReNu; LivaNova’s OSPREY Trial Achieves Key Safety and Efficacy Milestones; Hyperfine Launches Advanced Portable MR Brain Imaging Swoop® System Across Europe; Nihon Kohden Strengthens Neurological Solutions Through Ad-Tech Medical Instrument Corporation Acquisition
Caris Life Sciences Received FDA Approval for MI Cancer Seek™ as a Companion Diagnostic (CDx) Test On November 6, 2024, Caris Life Sciences, a leading next-generation AI TechBio company and precision medicine pioneer, announced that the U.S. Food and Drug Administration (FDA) approved MI Cancer Seek™ for u...
Read More...
Sep 05, 2024
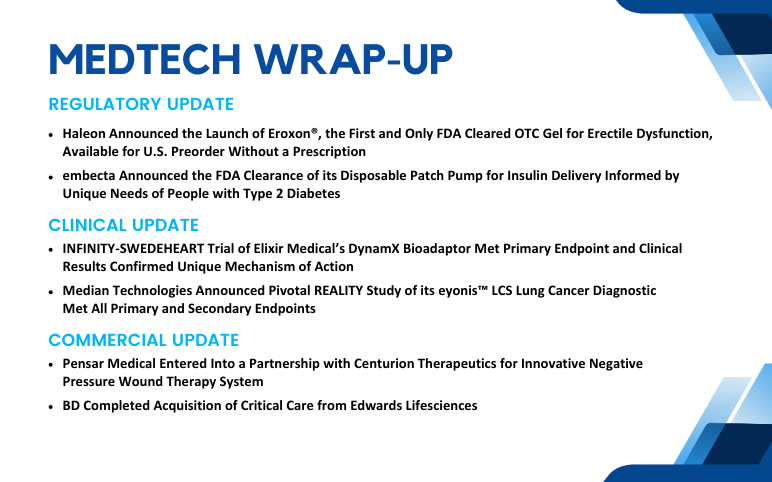
Pensar Medical’s Partnership with Centurion Therapeutics; BD’ Acquisition of Critical Care; Haleon’s Launch of Eroxon; embecta Receives FDA Clearance; INFINITY-SWEDEHEART Trial of Elixir Medical’s DynamX Bioadaptor; Median Technologies’ Pivotal REALITY Study
Pensar Medical Entered Into a Partnership with Centurion Therapeutics for Innovative Negative Pressure Wound Therapy System On Aug 29, 2024, Pensar Medical, a pioneer in negative pressure wound therapy (NPWT), announced a strategic partnership with Centurion Therapeutics to distribute the Microdoc® sNPWT (...
Read More...
May 09, 2024
Monteris Smallest Brain Laser Probe Launch; Vesica Health’s AssureMDx Test Launch; Anaut’s Eureka α Japanese Regulatory Approval; Geneoscopy’s Labcorp-Partnered Colon Cancer Test FDA Approval; Novel Omeza® OCM™ Diabetic Foot Ulcers Results; LEO Pharma Phase III Plaque Psoriasis Trial Results
Monteris Launched Smallest Brain Laser Probe on the Market On May 01, 2024, Monteris Medical launched the NeuroBlate® NB3™ FullFire® 1.6mm laser probe, the company’s latest product line innovation for use with their market-leading NeuroBlate System. The NB3 laser probe, which integrates Monteris' patented coo...
Read More...
Nov 30, 2023
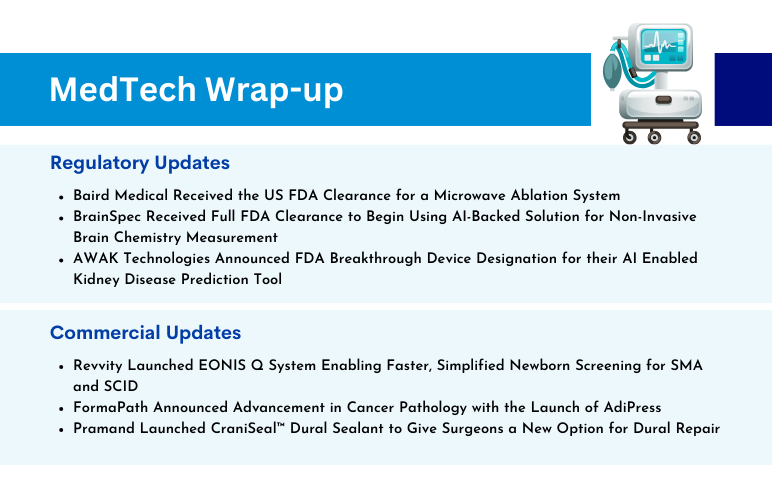
Baird Medical’s Microwave Ablation System; BrainSpec’s AI-Backed Solution for Non-Invasive Brain Chemistry Measurement; AWAK’s AI-Enabled Kidney Disease Prediction Tool; Revvity’s EONIS Q System; FormaPath Announced Advancement in Cancer Pathology; Pramand Launched CraniSeal Dural Sealant
Baird Medical Received the US FDA Clearance for a Microwave Ablation System On November 22, 2023, Baird Medical announced the US Food and Drug Administration clearance of the company’s microwave ablation (MWA) system and disposable needles for use in the US. The clearance allows the use of the technology to a...
Read More...
Sep 28, 2023
Orthofix Launched the Galaxy Fixation Gemini System; Oxford Biodynamics Launched the EpiSwitch Prostate Screening Blood Test; CE Mark for the Medtronic’s New Simplera CGM; FDA 510(k) Clearance to MicroVention’s SOFIA™ EX 5F 115cm; SHL Telemedicine’s SmartHeart® Technology; Cardiosense’s Nationwide Heart Failure Study
Orthofix Launched the Galaxy Fixation Gemini System and Expanded Sterile Kit Offerings for Orthopedic Trauma Procedures On September 20, 2023, Orthofix Medical Inc., a leading global spine and orthopedics company, launched the Galaxy Fixation Gemini™ system. It is a stable external fixation system that ...
Read More...
Aug 10, 2023
Quest Introduced Consumer-Initiated Blood Test for Alzheimer’s Disease Risk Assessment; DuPont Acquires Spectrum Plastics; BD Received 510(k) Clearance for Molecular Combination Test; Natera Updates on Phase III ALTAIR Trial of Signatera; Anteris’ DurAVR transcatheter heart valve Trial
Quest Introduced First-To-Market Consumer-Initiated Blood Test for Alzheimer's Disease Risk Assessment on Questhealth Website On July 31, 2023, Quest Diagnostics, a leader in diagnostic information services, announced the introduction of the AD-Detect™ Test for Alzheimer's Disease on questhealth.com – the first ...
Read More...





-Agonist.png)

