CAR-T
Jun 10, 2025
Bispecific and Trispecific Antibodies: Are They Better Than CAR-Ts?
CAR-T cell therapy has revolutionized the treatment of blood cancers, earning regulatory approval and delivering impressive results in hematologic malignancies. However, its success has not yet translated to solid tumors, where it continues to face significant challenges and limited long-term efficacy. As a result,...
Read More...
Oct 21, 2024
Exosomes: Tiny Messengers with Big Potential in Medical Science
Exosomes are tiny, powerful messengers that are revolutionizing the way we think about communication between cells. These nanosized vesicles carry crucial signals—proteins, lipids, and RNA—between cells, playing a pivotal role in everything from immune responses to tissue repair. What makes exosomes truly exciting ...
Read More...
Sep 02, 2024
CAR T-Cell Therapies in Non-Hodgkin’s Lymphoma Treatment: A Revolutionary Approach
CAR-T cells offer a lasting benefit through a single treatment, sparing patients with high-risk conditions from the toxicity associated with salvage chemotherapy and autologous transplants. These approvals have changed the standard of care for patients who are either resistant to initial treatment or experience ear...
Read More...
May 21, 2024
Amgen’s IMDELLTRA FDA Approval; J&J’s Proteologix Acquisition; Bristol Myers Squibb’s BREYANZI FDA Approval; AbbVie and Gilgamesh Pharmaceuticals’ Agreement; Eisai’s LEQEMBI FDA Fast Track Status
IMDELLTRA Receives FDA Approval as the First T-Cell Engager Therapy for Advanced Small Cell Lung Cancer Amgen has reported that the FDA has approved IMDELLTRA™ (tarlatamab-dlle) for treating adult patients with extensive-stage small cell lung cancer (ES-SCLC) who have experienced disease progression following pl...
Read More...
Sep 13, 2022
Amgen Announced the Result of its CodeBreak-200 Trial; FDA Clears Bristol-Myers Squibb’s Deucravacitinib; BioNTech’s Amplified CAR-T Therapy; TIL Therapy Improves on Yervoy in Melanoma; GSK’s Daprodustat will have to face FDA Advisory Committee; Breakthrough Therapy Status to Pfizer’s Group B Strep Vaccine; EU Approves Gilead’ Tecartus; Gilead’ Trodelvy Results in TROPiCs-02 Trial
Amgen Reveals the Top-line Result of its CodeBreak-200 trial of Lumakras in Lung Cancer The top-line result of Amgen's CodeBreak-200 trial of Lumakras in lung cancer was presented in abstract form at ESMO two weeks ago, showing a 34% improvement in progression-free survival (PFS) compared to chemotherapy. The fu...
Read More...
Aug 16, 2022
Novartis’ Canakinumab for NSCLC; Novartis’s Zolgensma Updates; Trodelvy Prospects in New Breast Cancer Use; Novartis Secures European Approval For Pluvicto; Bristol-Myers Squibb’s Abecma Phase III Trials; Trastuzumab Approved for Deruxtecan for HER2-Mutant NSCLC
Novartis’ Canakinumab Fails in Phase III Trials for NSCLC Canakinumab's prospects as an anticancer therapy were already dwindling when a third phase III trial in non-small cell lung cancer (NSCLC) failed to reach its objectives. The most recent setback came from the CANOPY-A study, which included patients with N...
Read More...
May 24, 2022
PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboration with Atara; FDA Accepts Biohaven’s Zavegepant; AbbVie Files FDA Approval for ABBV-951; Innoviva to Acquire Entasis; FDA Orphan Drug Designation to XMT-2056; FDA Approves Azacitidine for Juvenile Myelomonocytic Leukemia
EU Recommends Approval for PTC Therapeutics’ Gene Therapy Upstaza Upstaza, a gene therapy developed by PTC Therapeutics for patients with the genetic condition AADC deficiency, has been recommended for EU approval, putting another test of gene therapy's commercial prospects in the union. Upstaza (eladocagene exu...
Read More...
Feb 22, 2022
Sandoz’s Generic Revlimid; Agios’ Pyrukynd; Organon Announces 4Q & Full-year Earnings Report; BMS’ CAR-T Drug Breyanzi; Sanofi & Regeneron’s Dupixent Trial; AZ & Daiichi’s Drug Enhertu; Bayer’s Drug Kerendia; Lilly Releases Mirikizumab Data
Sandoz Launches generic Revlimid in 19 European Countries, Bringing a Flood of Competition to BMS' Megablockbuster Since Bristol Myers Squibb acquired Celgene and its megablockbuster Revlimid, the company has been bracing for the day when the multiple myeloma superstar would face generic competition. Sandoz, a s...
Read More...
Dec 14, 2021
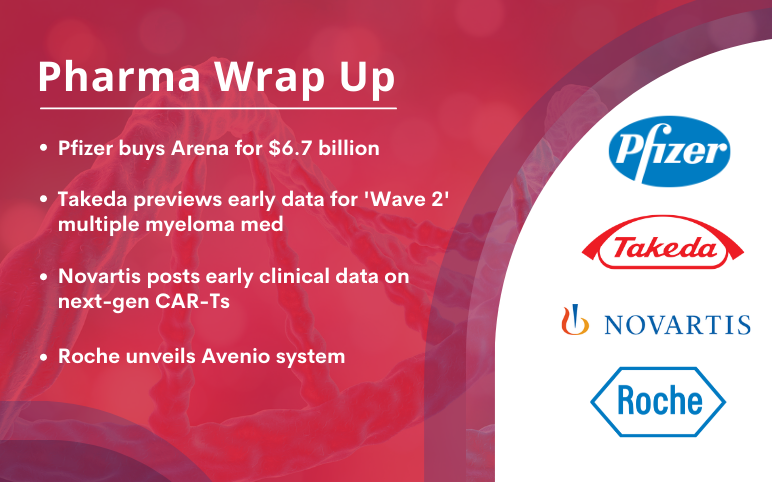
Pfizer to acquire Arena Pharma; Takeda’s ‘Wave 2’ multiple myeloma med data; Novartis’s next-gen CAR-Ts clinical data; Roche unveils Avenio system
Pfizer acquires drugmaker Arena for USD 6.7 billion to address unmet needs Pfizer declared that it is acquiring Arena Pharmaceuticals, a drug company specializing in inflammation and immunology, for almost USD 7 billion. The pharma giant said it would finance the agreement, worth USD 6.7 billion, with cash on ha...
Read More...
May 07, 2020
Avrobio taps Magenta’s ADC; Eli Lilly ramps up; FDA postpones decision; CSL gears up to fight pandemic,
Avrobio taps ADC of Magenta for enhancing gene therapy conditioning Avrobio is working to make conditioning safer. The company is collaborating with Magenta Therapeutics to check antibody-drug conjugate. Under the agreement, the partners will evaluate Magenta’s lead conditioning program, MGTA-117, with at lea...
Read More...







-Agonist.png)

