Deep brain stimulation Devices
Oct 28, 2025
Wireless Brain Sensors: Revolutionizing Neuroscience and Healthcare
Wireless brain sensors are emerging as a transformative technology in neuroscience and healthcare, offering a new level of precision, comfort, and real-time monitoring for patients with neurological conditions. Unlike traditional wired sensors that limit mobility and can cause discomfort, wireless brain sensors use...
Read More...
Oct 16, 2025
Scanogen’s Bloodstream Infection Assay Earns FDA Breakthrough Device Status; Lapsi Health Launches Keikku 2.0; Medtronic BrainSense™ Adaptive DBS Earns 2025 TIME Best Inventions Honor; Photocure Partners with Intelligent Scopes Corporation to Advance AI Integration in Blue Light Cystoscopy; Conformal Medical Expands CONFORM Trial to Europe; Materna Medical Presents Study on At-Home Patient Outcomes: Experienced vs. Naïve Dilator Users
Scanogen Received FDA Breakthrough Device Designation for Rapid Bloodstream Infection Assay On October 15, 2025, Scanogen Inc., a molecular diagnostics innovator developing next-generation infectious disease detection technologies, announced that the U.S. Food and Drug Administration (FDA) granted Breakthr...
Read More...
Mar 05, 2025
Major Depressive Disorder: Unveiling Market Moves and Commercial Breakthroughs
Major Depressive Disorder (MDD) is a widespread and debilitating psychiatric condition that continues to challenge patients, caregivers, and healthcare systems worldwide. Characterized by persistent sadness, loss of interest, cognitive dysfunction, and suicidal ideation, MDD remains a major public health concern. D...
Read More...
Feb 27, 2025
Medtronic Secures FDA Approval for First-Ever Adaptive Deep Brain Stimulation System for Parkinson’s; FDA Grants Breakthrough Device Designation to Averto Medical’s ColoSeal™ System; Biomerica’s inFoods® IBS clinical trial findings were published in Gastroenterology; Lungpacer Medical’s STIMULUS Trial Highlights Improved Hemodynamics With Diaphragm Neurostimulation; Alcon Introduces Voyager DSLT; Stryker Finalizes Acquisition of Inari Medical
Medtronic Earned U.S. FDA Approval for the World's First Adaptive Deep Brain Stimulation System for People With Parkinson's On February 24, 2025, Medtronic plc, a global leader in healthcare technology, announced that it had received U.S. Food and Drug Administration (FDA) approval for BrainSense™ Adaptive...
Read More...
Jan 16, 2025
Johnson & Johnson MedTech Secures CE Mark for Dual Energy THERMOCOOL SMARTTOUCH™ SF Catheter; Medtronic Secures CE Mark for BrainSense™ Adaptive Deep Brain Stimulation; Inquis Medical Finalizes Enrollment for U.S. Pivotal Study on Aventus Thrombectomy System for Pulmonary Embolism; First Patient Enrolled in Argon Medical’s CLEAN-PE Study for Novel Pulmonary Embolism Therapy; Sutter Health and GE HealthCare Partner to Revolutionize Patient Care with AI-Driven Imaging Solution; Saluda Medical Closes $100M Financing to Expand Neuromodulation Portfolio
Johnson & Johnson MedTech Announced CE Mark Approval for Dual Energy THERMOCOOL SMARTTOUCHTM SF Catheter, Bolstering Capabilities in Cardiac Arrhythmias Treatment On January 10, 2025, Johnson & Johnson MedTech, a global leader in cardiac arrhythmia treatment, announced the receipt of European CE ma...
Read More...
May 18, 2023
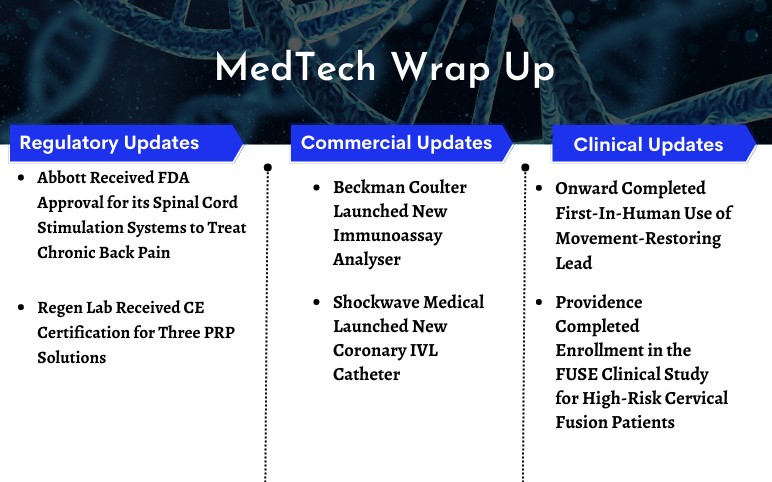
FDA Approves Abbott’s Spinal Cord Stimulation Systems; Regen Lab Received CE Certification for Three PRP Solutions; Beckman Coulter Launched New Immunoassay Analyser; Shockwave Medical’s Coronary IVL Catheter; Providence Completed Enrollment in the FUSE Clinical Study; Onward Completed First-In-Human Use of Movement-Restoring Lead
Providence Medical Technology Completed Enrolment in the FUSE Clinical Study for High-Risk Cervical Fusion Patients On May 11, 2023, Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, closed the enrolment in its FUSE Study which is a prospective, multicenter...
Read More...
Feb 23, 2023
Medtronic’s Extravascular Defibrillator System; Stryker’s Q Guidance System for Cranial Applications; Synchrony Medical’s LibAirty™ Airway Clearance System Study; Cognito Therapeutics’s Pivotal Study HOPE Update; Dexcom’s G6 Continuous Glucose Monitoring System Launch in Singapore; Rhaeos Raised $10.5M in Series A Funding
Medtronic Received CE Mark for Extravascular Defibrillator System, used to treat Abnormal Heart Rhythms On February 17, 2023, Medtronic plc, the leading global healthcare technology company, received CE mark for the Aurora EV-ICD™ MRI SureScan™ (Extravascular Implantable Cardioverter-Defibrillator)...
Read More...
Mar 26, 2021
How Will New Discoveries of Deep Brain Stimulation Devices Transform the Pharma Domain?
Deep brain stimulation (DBS) is a surgical procedure that requires the implantation of a device capable of sending electrical impulses to the brain areas responsible for body movement. A DBS system is composed of the neuro-stimulator, leads, and extension. The electrodes are placed deep inside the brain and are lin...
Read More...






-Agonist.png)

