Digital Health
Oct 13, 2025
Digital Twins in the Healthcare Industry
Digital twins are advanced digital replicas of physical systems, processes, or entities that use real-time data, artificial intelligence (AI), machine learning (ML), and the Internet of Things (IoT) to simulate, monitor, and predict the behavior of their real-world counterparts. In healthcare, digital twins can rep...
Read More...
Jun 06, 2025
Assessing the Growing Role & the Demand of Chronic Disease Management Apps
Over the past few years, the prevalence of chronic diseases has grown significantly, placing an increasing social and economic burden on individuals and families. Managing these long-lasting conditions requires constant attention to symptoms, medication, lifestyle changes, and emotional well-being. As a result, chr...
Read More...
Dec 18, 2024
Mobile Clinics: Bridging the Healthcare Gap
Access to healthcare is a fundamental human right, yet millions of people around the world face significant barriers to receiving timely and effective medical attention. Whether due to geographic isolation, economic disparities, or a shortage of healthcare facilities, these barriers can lead to worsening health out...
Read More...
Oct 10, 2024
Johnson & Johnson Acquires V-Wave; CalmiGo Launches Anxiety-Management Platform CalmiGo Plus; Vy Spine Secures FDA Clearance for 3D-Printed Lumbar IBF; InspireMD Receives IDE Approval for CGuard Stent Study; GE Healthcare Reveals Phase I MRI Contrast Results; Medinol Completes First Human Implantation of Drug-Eluting Stent
Johnson & Johnson Completed Acquisition of V-Wave On Oct 09, 2024, Johnson & Johnson announced that it successfully completed the acquisition of V-Wave Ltd., a privately-held company dedicated to developing innovative treatments for heart failure patients. V-Wave will now operate under Johnson & John...
Read More...
Jul 18, 2024
Inspira™ Approval of INSPIRA™ ART100 System; Oticon Medical’s Sentio™ System Received Regulatory Clearance; SpineGuard Filed its “510K” Dossier in the US; Vectorious Medical Technologies’ V-LAP Left Atrial Pressure Sensor Successful Implantation; Bedal International Raised $11 Million; Mytonomy Inc. Agreement With Vizient, Inc.
Inspira™ Secured Approval From Israeli Authorities for its INSPIRA™ ART100 System On July 11, 2024, Inspira™ Technologies OXY B.H.N. Ltd., a pioneering medical technology company, secured a pivotal milestone with the receipt of the Israeli Ministry of Health's medical devices and accessories ("AMAR") approval fo...
Read More...
Jun 20, 2024
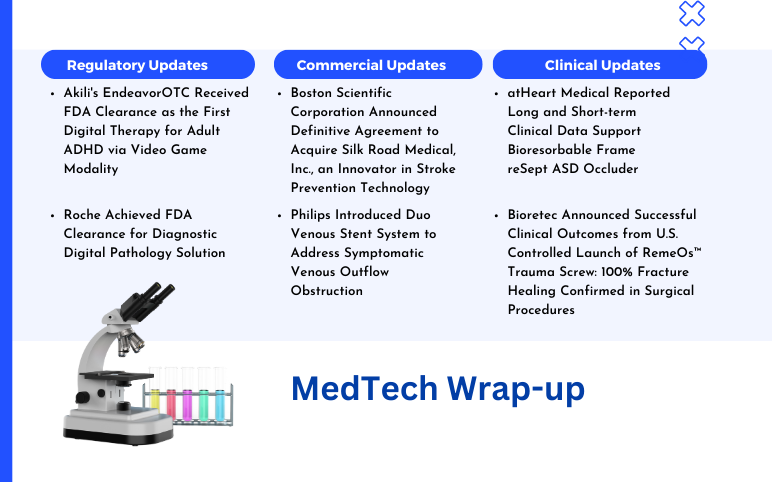
Boston Scientific Corporation Agreement to Acquire Silk Road Medical; Philips Introduced Duo Venous Stent System; Akili’s EndeavorOTC Received FDA Clearance; Roche Achieved FDA Clearance; atHeart Medical Reported Long and Short-term Clinical Data; Bioretec Announced Successful Clinical Outcomes
Boston Scientific Corporation Announced Definitive Agreement to Acquire Silk Road Medical, Inc., an Innovator in Stroke Prevention Technology On June 18, 2024, Boston Scientific Corporation, a global leader in medical technologies, announced the formal agreement to acquire Silk Medical, Inc., a medical device co...
Read More...
Feb 22, 2024
Johnson & Johnson’s Tecnis PureSee lens; Sparrow BioAcoustics’s Stethoscope Software; Better Therapeutics’s MASH Treatment; X-trodes’ Wearable “Skin” Solution; Lumicell’s LUMISIGHTTM; Konesksa Enrolled First Patient in Learns Observational Study
Johnson & Johnson, Launched New Intraocular Lens in EMEA Region On February 15, 2024, Johnson & Johnson Medtech’s vision unit launched the Tecnis PureSee lens in the EMEA region. Tecnis PureSee is a purely refractive intraocular lens (IOL) specifically designed to correct presbyopia. Its proprietary desi...
Read More...
Nov 23, 2023
Ethicon’s ETHIZIA Hemostatic Sealing Patch; FDA Approves Medtronic’s Minimally Invasive Device to Treat Hypertension; Boston Scientific Acquires Relievant Medsystems; AstraZeneca Launched Evinova; Surmodics Announced Data of the SWING Trial; GSK Advancing the Low Carbon Ventolin Program to Phase III Trials
Ethicon Announced European Approval and Introduction of ETHIZIA™ Hemostatic Sealing Patch Used to Stop Disruptive Bleeding On November 15, 2023, Ethicon, a Johnson & Johnson MedTech company, received approval for ETHIZIA™, an adjunctive hemostat solution that has been clinically proven to achieve sustained h...
Read More...
Jul 27, 2023
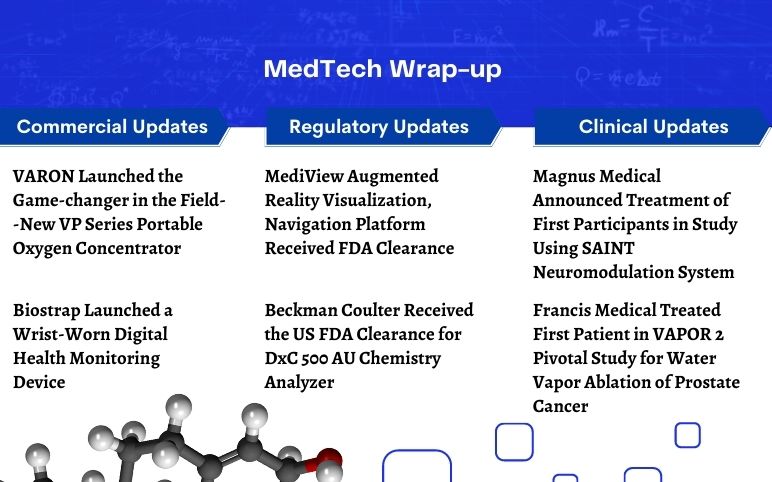
VARON’s New VP Series Portable Oxygen Concentrator; Biostrap’s Wrist-Worn Digital Health Monitoring Device; MediView’s AR Navigation Platform; Beckman Coulter’s DxC 500 AU Chemistry Analyzer; Magnus Updated on Study Using SAINT Neuromodulation System; Francis Medical’s VAPOR 2 Pivotal Study
VARON Launched the Game-changer in the Field--New VP Series Portable Oxygen Concentrator On July 19, 2023, VARON, a leading oxygen concentrator manufacturer, announced the launch of a game-changer portable oxygen concentrator VP-2. A new member of the VP series, VP-2 is incorporated with innovative tech...
Read More...
Jul 20, 2023
Relievant Medsystems Launched Ablation System; Thermo Fisher’s Reproductive Health Assays; EarliTec’s Next-Generation EarliPoint Evaluation for ASD; FDA Cleared ReddyPort® Non-Invasive Ventilation Device; EndoTheia’s Novel Medical Device for Endoscopic Surgery; Neurolief’s MOOD Trial Interim Analysis
Relievant Medsystems Launched New Tools for its Ablation System On June 14, 2023, Relievant Medsystems announced that it launched its next-generation access instruments for the Intracept procedure. Chronic vertebrogenic low back pain is treated with Intracept, a minimally invasive, same-day, outpatient proced...
Read More...





-Agonist.png)

