FDA
Jun 22, 2017
Novartis on AMD drug; Hospira recall of vials; Takeda wraps up; FDA bans imports
Novartis takes on Regeneron with AMD drug that needs fewer doses than Eylea Last year, U.S. sales of Regeneron’s drug to treat age-related macular degeneration (AMD) came in at $3.3 billion—68% of the company’s total revenues—so it’s no wonder investors have been expressing some concerns about potential rivals movin...
Read More...
May 26, 2017
Progressive Supranuclear Palsy– A neurodegenerative disorder
Steele-Richardson-Olszewski syndrome, commonly known as Progressive Supranuclear Palsy, is an uncommon brain disorder that causes serious problems for patients in terms of walking, balance and eye movements. The disorder arises as a result of deterioration of cells in areas of the brain that control body movement an...
Read More...
May 02, 2017
FDA Approves; AZ nabs; Nordisk settles; Novartis pays
FDA Approves Alunbrig (brigatinib) for Rare Lung Cancer Takeda Pharmaceuticals announced the FDA approved Alunbrig (brigatinib) to treat patients with anaplastic lymphoma kinase-positive (ALK+) metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib. Brigatinib is a kinas...
Read More...
Apr 12, 2017
Dengvaxia study; Opdivo racks up; FDA issues plant; Teva’s Rimsa fraud; Mission bags Fox grant
Malaysia calls for phase 4 Dengvaxia study before considering full approval Already struggling to meet initial expectations, Sanofi Pasteur's dengue vaccine Dengvaxia will have to undergo phase 4 testing in Malaysia before the endemic country agrees to sign off on a full approval. Malaysia’s National Pharmaceutical ...
Read More...
Mar 28, 2017
Tesaro puts AstraZeneca; XELJANZ® receives; Innovus nabs; Alexion brings
Tesaro puts AstraZeneca on notice with early FDA nod for Lynparza rival niraparib Tesaro’s closely watched ovarian cancer drug niraparib—now dubbed Zejula—won an FDA nod on 27th March, months before its scheduled decision date. Apart from getting approval, Zejula also got a broader label than its head-to-head rival,...
Read More...
Feb 28, 2017
Prosecutors rope Pfizer; Pharma groups to FDA; Regeneron simulates; Otsuka & Lundbeck revive;Philidor execs plead
Prosecutors rope Pfizer into fast-growing copay assistance probe After first focusing on biotech and speciality pharma, the feds have made their way to Big Pharma, with the New York drug giant joining a group that includes Gilead Sciences, Biogen, Valeant Pharmaceuticals and others about ties to copay assistance pro...
Read More...
Jan 27, 2017
Payers block EpiPen; Allergan charged; AbbVie’s Humira; Baxter paying $18M
Payers block Kaléo's expensive EpiPen challenger Kaléo reintroduced its Auvi-Q last week at a list price of $4,500 for a two-pack in an effort to capture some market share from Mylan’s EpiPen, which is listed at about $600 for a two-pack. Auvi-Q is set to launch next month. Under Kaléo’s pricing strategy, Auvi-Q wil...
Read More...
Dec 20, 2016
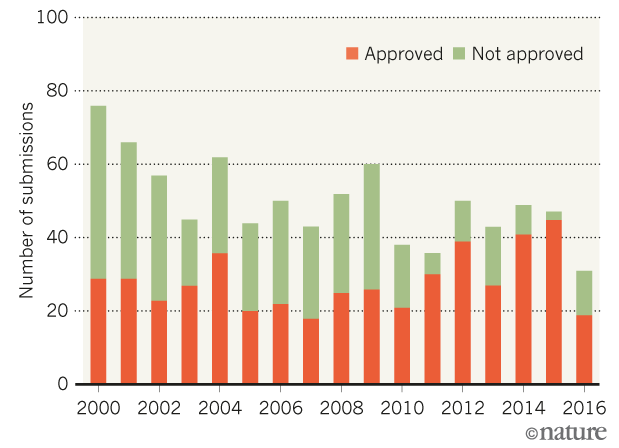
US drug approvals plummet in 2016
US drug approvals are on track to drop by more than half in 2016 compared to 2015. The agency had approved 19 new drugs this year as of 9 December, putting it on track for its lowest yearly tally since 2007. The decline is made more dramatic by 2015’s bumper crop of approvals. The FDA approved 45 new drugs last year...
Read More...
Nov 29, 2016
EC grants; Pfizer cuts; 27 medicines sold; Keytruda nabs
EC grants marketing authorisation for Takeda’s Ninlaro capsules The European Commission (EC) has granted conditional marketing authorisation for Takeda Pharmaceutical’s Ninlaro capsules. The oral proteasome inhibitor is indicated in combination with lenalidomide and dexamethasone for adult patients with multiple mye...
Read More...
Oct 04, 2016
FDA Approves STELARA; Novartis announces AMG 334; AbbVie’s HCV Regimen; PaizaBio Gains CFDA Approval; WuXi Biologics completes construction
FDA Approves Janssen's STELARA for the Treatment of Adults With Moderately to Severely Active Crohn’s Disease Janssen Biotech Inc. received approval from the U.S. FDA for STELARA (ustekinumab), used for the treatment of moderately to severely active Crohn’s disease in adults (18 years or older). The drug is for the ...
Read More...






-Agonist.png)

