Idiopathic Pulmonary Fibrosis
Oct 14, 2025
FDA Approves Boehringer’s JASCAYD as First New IPF Therapy in Over a Decade; Denali Therapeutics’ Tividenofusp Alfa BLA Review for Hunter Syndrome Extended by FDA; Bicara’s Ficerafusp Alfa Earns FDA Breakthrough Tag for 1L HPV-Negative Head & Neck Cancer; BeOne Medicines’ Sonrotoclax Granted FDA Breakthrough Designation for Cancer Treatment; FDA Clears LIBTAYO for Adjuvant Use in Cutaneous Squamous Cell Carcinoma
FDA Approves Boehringer’s JASCAYD as First New IPF Therapy in Over a Decade Boehringer Ingelheim’s JASCAYD (nerandomilast) has received FDA approval as an oral treatment for adults with idiopathic pulmonary fibrosis (IPF), marking the first new therapy for the condition in over a decade. JASCAYD is the first and...
Read More...
Oct 10, 2025
Boehringer Ingelheim’s JASCAYD Brings Hope Back to IPF Patients After a Decade of Waiting
Over a decade after launching one of the first treatments for idiopathic pulmonary fibrosis, Boehringer Ingelheim is rejuvenating its presence in the rare lung disease arena with a newly approved therapy. On October 7, the FDA approved JASCAYD, marking the first new IPF treatment in over ten years. The approval fol...
Read More...
Jun 27, 2023
FDA Approves Jardiance for Type 2 Diabetes; FDA Approves Pfizer’s LITFULO for Alopecia Areata; Sarepta Therapeutics’s ELEVIDYS Approval; Tonix Pharmaceuticals to Acquire Two Migraine Products from Upsher-Smith; FibroGen’s Phase 3 ZEPHYRUS-1 Study of Pamrevlumab; FDA Orphan Drug Designation to ERAS-801 for Malignant Glioma
FDA Approves Jardiance for the Treatment of Type 2 Diabetes in Children 10 Years and Older Boehringer Ingelheim and Eli Lilly and Company announced that the FDA has approved Jardiance® (empagliflozin) 10 mg and 25 mg tablets to decrease blood sugar together with diet and exercise in children 10 years and older w...
Read More...
Nov 01, 2022
Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announces BLA Submission for SER-109; BMS Announces Results of COMMANDS Trial; Boehringer’s PDE4B Moves Late-stage Clinical Testing; FDA Rejects Gilead’s Hepcludex; Approval to J&J’s BCMAxCD3 Bispecific Antibody for Multiple Myeloma; Syncona to Acquire AGTC
Actinium Announces Positive Top-line Results from Pivotal Phase III SIERRA Trial of Iomab-B Actinium Pharma is on track to submit its targeted radiotherapy for AML patients requiring a bone marrow transplant in the United States, boosted by top-line data from a pivotal trial. The SIERRA trial of Iomab-B, an anti...
Read More...
Oct 25, 2022
Sumitomo to Purchase Myovant; AstraZeneca’s Tremelimumab Plus Imfinzi Approved in the US; AbbVie Acquires DJS Antibodies; Roche and Hookipa Pharma Signs USD 25 Million Deal; FDA Accepts BMS’s New Drug Application for CAMZYOS; Jazz and Zymeworks Sign Exclusive Licencing Agreement
FDA Accepts Bristol Myers Squibb’s Supplemental New Drug Application for CAMZYOS Bristol Myers Squibb declared that the U.S. FDA had accepted its supplemental new drug application for CAMZYOS for an expanded indication to reduce the need for septal reduction therapy. CAMYZOS is currently FDA-approved for treatin...
Read More...
Sep 26, 2022
PDE4-B Inhibitors: A Promising Target for Idiopathic Pulmonary Fibrosis Treatment
Idiopathic pulmonary fibrosis (IPF) is a rare, sporadic, and fatal interstitial lung disease. As the morbidity and mortality rates associated with IPF remain high, prompt idiopathic pulmonary fibrosis treatment is critical to safeguard individuals’ lung function, reduce the risk of acute exacerbations, and improve ...
Read More...
May 17, 2022
BMS Sells NY Biologics Plant; FDA Approves Mitsubishi Tanabe’s Radicava, Love Pharma Acquires MicroDoz; Sandoz Launches Generic of Roche’s Esbriet; Pfizer to Acquire Biohaven Pharma; eureKARE’s Cell and Gene Therapy CDMO; Atamyo’s LGMD Gene Therapies; FDA approves Eli Lilly’s Type 2 Diabetes Treatment
Love Pharma Completes the Acquisition of MicroDoz Therapy LOVE Pharma Co. has announced the completion of its acquisition of MicroDoz Theraphy Inc. ("MicroDoz"). Love Pharma holds exclusive manufacturing, marketing, packaging, selling, and distribution licenses in Europe, the United Kingdom, and North America. U...
Read More...
Apr 26, 2022
Coherus Biosciences’ Toripalimab; Keymed’s CMG901; Biogen Alzheimer’s Drug Aduhelm; Vicore’s Digital Therapeutics Study; Cassiopea’s Acne Treatment Winlevi; Samsung Biologics Acquires Samsung Bioepis; Roche’s Giredestrant; Ashai Kasei Acquires Bionova
FDA Grants Orphan Drug Designation to Coherus Biosciences’ Toripalimab for Small Cell Lung Cancer The FDA granted orphan drug status to Coherus Biosciences’ Toripalimab (TuoYi) based on data from the phase 3 JUPITER-08 study in patients with extensive-stage Small-cell Lung Cancer (ES-SCLC). Patients in the curre...
Read More...
Jan 18, 2022
Algernon’s NP-120 (ifenprodil); Pieris’s cinrebafusp alfa (PRS-343) clinical trial; Gaumard Scientific’s multidisciplinary patient simulator HAL S5301; Hekka Labs’s decentralized healthcare ecosystem
Algernon receives positive FDA feedback on Phase IIb chronic cough trial The US Food and Drug Administration (FDA) has given positive feedback for Algernon Pharmaceuticals’ Phase IIb clinical trial of NP-120 (ifenprodil) for chronic cough treatment. A receptor antagonist of N-methyl-D-aspartate (NMDA), ifenp...
Read More...
Mar 11, 2020
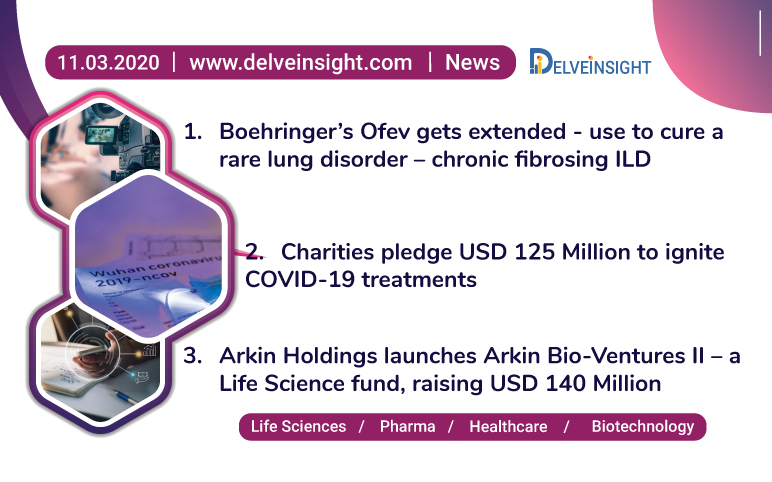
Ofev’s expanded use; Arkin Bio-Ventures II launch; USD 125 M for COVID-19 treatment
Boehringer Ingelheim’s Ofev (nintedanib) has received FDA recommendation for the treatment of chronic fibrosing interstitial lung diseases (ILD) with a progressive phenotype Interstitial Lung Disease - ILD – a group of a large number of lung disorders resulting in scarring or fibrosis of lungs, caused by conditi...
Read More...







-Agonist.png)

