Mammography Devices
Aug 21, 2025
FDA Grants 510(k) Clearance to Nerveblox AI; Labcorp Introduces the First FDA-Cleared Alzheimer’s Blood Test; Calidar Introduces First-of-its-Kind 4D Mammography System; ONWARD Medical to Initiate Empower BP Pivotal Study Following FDA IDE Approval; Tenon® Medical Launches Catamaran® SE SI Joint Fusion System Nationwide; Rivanna Secures $800,000 Virginia Catalyst Grant Plus Matching Funds to Advance AI-Driven Ultrasound Innovation
Nerveblox, the Ultrasound AI for Regional Anesthesia Received FDA Clearance On August 18, 2025, Nerveblox, an AI-powered software developed by SmartAlpha to assist physicians in performing ultrasound-guided regional anesthesia, received U.S. Food and Drug Administration (FDA) 510(k) clearance. This milesto...
Read More...
Nov 28, 2024
FDA Clears Medtronic’s New Inpen™ App; Zimmer Biomet Gets FDA Approval for Oxford® Cementless Partial Knee; Perimeter B-Series OCT with ImgAssist AI 2.0 Shows Significant Superiority Over Standard-of-Care in Pivotal Clinical Trial; Edwards’ SAPIEN 3 Ultra RESILIA Valve Maintains Exceptional Patient Outcomes in Real-World Settings; Abbott Introduces AVEIR™ VR Leadless Pacemaker System to Indian Market; GE HealthCare’s Pristina Via Transforms Mammography with Improved Workflow and Patient Care
Medtronic Received FDA Clearance for the New Inpen™ App, Paving the Way for its Smart MDI System Launch with Simplera™ CGM On November 20, 2024, Medtronic, a global leader in healthcare technology, announced the U.S. Food and Drug Administration (FDA) clearance for its new InPen™ app, which includes advanc...
Read More...
May 30, 2024
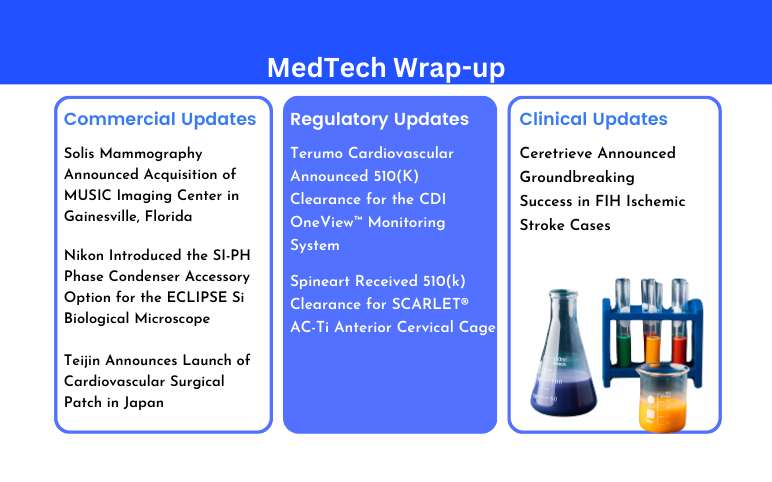
Solis Mammography’s Acquisition of MUSIC Imaging Center; Nikon’s SI-PH Phase Condenser Accessory Option; Teijin Launch Surgical Patch; Terumo Cardiovascular’s FDA 510(K) Clearance; Spineart’s 510(k) Clearance; Ceretrieve’s Success in FIH Ischemic Stroke Cases
Solis Mammography Announced Acquisition of MUSIC Imaging Center in Gainesville, Florida On May 28, 2024, Solis Mammography announced the acquisition of the MUSIC Mammography and Ultrasound Imaging Center in Gainesville, Florida. For more than 10 years, MUSIC has provided patients with an advanced standard ...
Read More...


-Agonist.png)

