Medical Devices Market
Aug 18, 2022
CereVasc’s eShunt System Study; FDA Approves NGS-Based CDx for Trastuzumab Deruxtecan; Nanopath Secures $10 Million Funding; BD, Accelerate Diagnostics Announce Collaboration; Avails Medical’s Clinical Trials for eQUANT; Movano Ring Exceeds Accuracy Targets for SpO2 & Heart Rate Monitoring
CereVasc Announces FDA Approval of Second IDE Study of the eShunt® System On August 09, 2022, CereVasc, Inc., a privately held, clinical-stage medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved ...
Read More...
Aug 17, 2022
Interoperability Solutions in Healthcare – Evolving Market Dynamics and Improving Health Outcomes
In today’s highly dynamic and evolving market dynamics, interoperability plays a key role in facilitating organized and effective data exchange between information systems. Businesses across different verticals and sectors are implementing interoperability in the system to address critical issues such as duplicate ...
Read More...
Jul 28, 2022
AbbVie & iSTAR’S Strategic Alliance; Baxter Launches Welch Allyn RetinaVue 100 Imager PRO; Instylla’S Embrace Hydrogel Embolic System; Primary Endpoint Met in the RADIANCE II US Trial; Roche’s Elecsys Amyloid Plasma Panel; EU Approval to Seegene’s Allplex; SARS-CoV-2/FluA/FluB/RSV Assay
ReCor Medical and Otsuka Medical Devices Announce Primary Endpoint Met in the RADIANCE II US Pivotal Trial of the Paradise™ System for the Treatment of Hypertension On July 26, 2022, ReCor Medical, Inc., a completely owned subsidiary of Otsuka Medical Devices Co., Ltd., with its headquarters in Palo Alto,...
Read More...
Jul 07, 2022
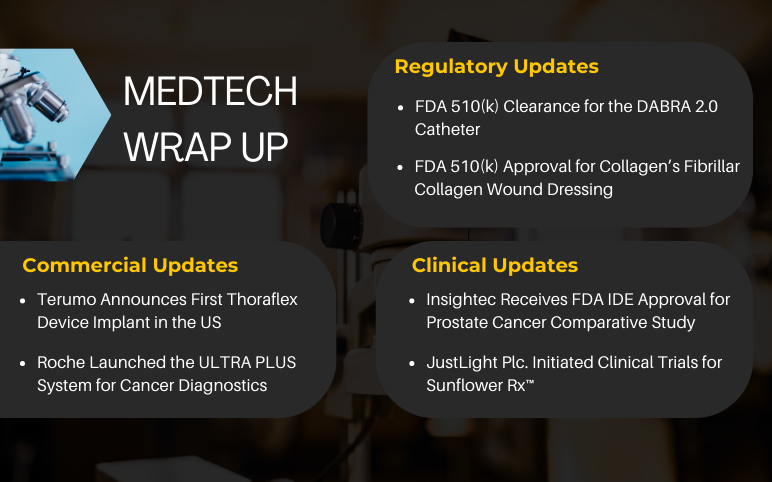
Collagen Matrix FDA 510(k) approval for Fibrillar Collagen Wound Dressing; Roche’s cancer diagnostics ULTRA PLUS system launch; Insightec IDE Approval for Prostate Cancer; JustLight plc. trial of Sunflower Rx for Alzheimer’s disease; Thoraflex Hybrid Device Implantation in the United States; FDA 510(k) Clearance for the DABRA 2.0 Catheter
Collagen Matrix received FDA 510(k) approval for Fibrillar Collagen Wound Dressing On June 29, 2022, Collagen Matrix, Inc., a leader in regenerative medicine, a global manufacturer of collagen and other biomaterial-based medical devices, and Linden Capital Partners portfolio company announced FDA 510(k) Clearan...
Read More...
Jun 08, 2022
How At-Home Medical Equipment is Improving the Quality of Medical Care?
An essential fact of today's healthcare system is that patients are released from hospitals due to increased stress even when they still need care. As a result, both the patient and professional caregivers utilize various technologies, including some complex mechanisms, in noninstitutional settings to manage their ...
Read More...
May 26, 2022
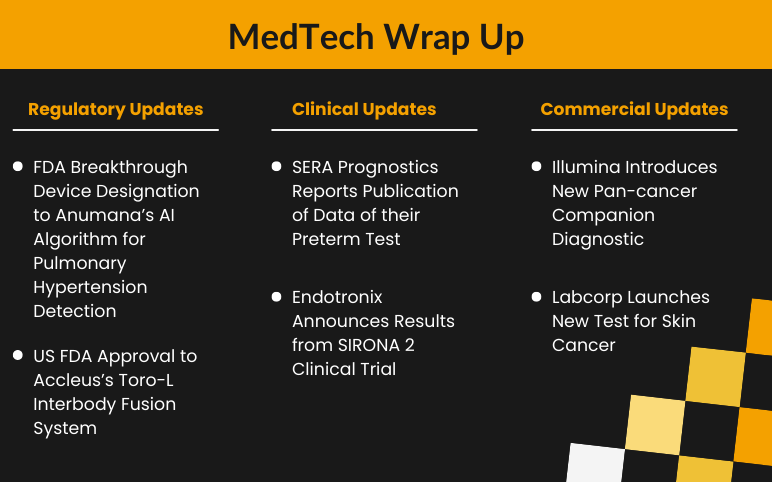
Accleus’s Toro-L Interbody Fusion System; Labcorp’s New Test for Skin Cancer; SERA Prognostics’s Preterm Test; Endotronix’s SIRONA 2 Clinical Trial; Anumana’s AI Algorithm to Detect ECG Pulmonary Hypertension; Illumina’s Pan-cancer Companion Diagnostic
Accleus Receives Regulatory Approval from US FDA for Toro-L Interbody Fusion System On May 19, 2022, the US Food and Drug Administration (FDA) granted the regulatory approval to Toro-L interbody fusion system developed by Accleus. The device is a biplanar expandable lateral implant designed for a minimal inserti...
Read More...
May 19, 2022
Check-Cap’s Pivotal Trial for C-Scan; Curebase and Flow Neuroscience’s tDCS Device; Brainlab Acquires Majority Share in medPhoton; GE Healthcare to Invest in Pulsenmore; Medtronic’s Drug-Eluting Coronary Stent System; Remedee’ Endorphin Stimulation Solution;
Check-Cap Initiated the US Pivotal Trial for C-Scan® On May 11, 2022, Check-Cap Ltd., a clinical-stage diagnostic company initiated the US pivotal trial for C-Scan® at Mayo Clinic in Rochester Minnesota. C-Scan is the first and only patient-friendly, preparation-free screening test to detect polyps before they m...
Read More...
Apr 29, 2022
Emerging Market for Organ on a Chip
The organ on a chip is a microfluidic culture device that recapitulates the complicated structures and functions associated with the living human organs. The microdevices are composed of a clear flexible polymer that is almost the size of a USB memory stick and comprises hollow microfluidic channels lined up with l...
Read More...
Apr 21, 2022
Penumbra’s Indigo System; Axonics’s F15 Recharge-free SNS; Stimwave’s FREEDOM-1 Clinical Trial; Virtual Incision’s MIRA Platform; SonoScape’s HD-550 Endoscopy system; SMART Medical’s G-EYE Colonoscope Series
Penumbra launched the Indigo® System with Lightning™ 7 and Lightning 12 Intelligent Aspiration in Europe On April 14, 2022, Penumbra, Inc., a leading medical device company focused on developing innovative therapies for neuro and vascular conditions, received the CE mark for its Indigo Aspiration System w...
Read More...
Mar 24, 2022
Smithfield BioScience and BioCircuit’s Nerve Tape Device; Cynosure-Jeisys Medical’s Partnership; bioMérieux’s VITEK® MS PRIME new MALDI-TOF Mass Spectrometry Identification System; Artio Medical’s Gold Embolization Device; Heartpoint Global’s Implant System; ObvioHealth’s Virtual Urogynecology Clinical Trial
Smithfield BioScience and BioCircuit to Develop New Nerve Tape Device On March 16, 2022, BioCircuit Technologies, a National Institutes of Health (NIH)-funded medical device company primarily focused on developing and commercializing tissue repair and neural interfacing products, and Smithfield BioScience, a uni...
Read More...








-Agonist.png)

