MedTech News
Mar 14, 2024
Allumiqs and Prolytix’s Partnership; Setpoint Medical’s Neuroimmune Modulation Platform gets FDA Breakthrough Designation; MangoRx Launches ‘PRIME’ with FDA-approved TRT; SeaStar Medical Updates Quelimmune Commercial Launch; Ventris Medical Gains 510(k) for Amplify® Bone Graft Putty
MangoRx Officially Launches ‘PRIME’ by MangoRx, Powered by Kyzatrex®️ FDA Approved Oral Testosterone Replacement Therapy (TRT) Treatment On March 12, 2024, Mangoceuticals, Inc. unveiled a groundbreaking development eagerly awaited by many: the launch of 'PRIME' by MangoRx, Powered by Kyzatrex®️. This release mar...
Read More...
Mar 07, 2024
Philips Teamed with SyntheticMR; BioPhotas’s Celluma Light Therapy; Boston Scientific’s AGENTTM Drug-Coated Balloon; Varian’s TrueBeam and Edge Radiotherapy Systems; Carthera’s Brain-Blood Barrier Trial; Prototype Device Effectively Treated Multiorgan Failure
Philips Teamed with SyntheticMR to Deliver Breakthrough AI-based Quantitative Brain Imaging in MR to Advance Neurology Care For Patients On March 01, 2024, Philips in collaboration with SyntheticMR announced the launch of Smart Quant Neuro 3D, a significant advancement in objective decision support for diag...
Read More...
Feb 22, 2024
Johnson & Johnson’s Tecnis PureSee lens; Sparrow BioAcoustics’s Stethoscope Software; Better Therapeutics’s MASH Treatment; X-trodes’ Wearable “Skin” Solution; Lumicell’s LUMISIGHTTM; Konesksa Enrolled First Patient in Learns Observational Study
Johnson & Johnson, Launched New Intraocular Lens in EMEA Region On February 15, 2024, Johnson & Johnson Medtech’s vision unit launched the Tecnis PureSee lens in the EMEA region. Tecnis PureSee is a purely refractive intraocular lens (IOL) specifically designed to correct presbyopia. Its proprietary desi...
Read More...
Feb 15, 2024
Tandem’s Automated Insulin Patch Pump; ResMed’s Bilevel Sleep Respiratory Devices; Masimo’s First Over-The-Counter Fingertip Pulse Oximeter; Viz.Ai Algorithm for Quantifying Intracerebral Hemorrhage; Canary Medical’s Heart Sound Monitor; UroMems’ Smart Urinary Sphincter System
Tandem Diabetes Care Launched Mobi Mini Automated Insulin Patch Pump On February 13, 2024, announced that it started off the U.S. commercial launch of its Mobi insulin patch pump. The San Diego-based business claims that Mobi, which is fully controllable via a smartphone app, is the world's smallest durable a...
Read More...
Feb 08, 2024
Cerenovus Launched Next-Gen Stroke Revascularization Catheter; Dexcom Launched New ONE+ CGM System; Philips Received the US FDA Nod for Latest Intellivue Patient Monitor Software; Toku Obtained CE and UKCA Marks for AI Cardiovascular Risk Assessments; Genesis MedTech Completed Enrollment in TAVR Trial; Carthera’s Ultrasound Drug Delivery Trial
J&J’s Cerenovus Launched Next-Gen Stroke Revascularization Catheter On February 7, 2024, Johnson & Johnson MedTech’s Cerenovus announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse indicated for the revascularization of patients suffering from acute ischemic stroke...
Read More...
Feb 01, 2024
Samsung Partnered with Lunit; Boston Scientific Launched VersaVue single-use Flexible Cystoscope; Element Science’s Patch-wearable Cardioverter Defibrillator; BIOCORP Received 510(k) FDA Clearance for SoloSmart; FDA IDE for CroiValve’s Tricuspid Heart Valve; FastWave Medical’s First-in-human Study of its IVL Tech
Samsung partnered with Lunit on AI for Enhanced Chest Screening On January 25, 2024, Samsung entered into a supply collaboration with Lunit, in order to employ its AI-powered technology for conducting chest screenings. The agreement was signed by Boston Imaging, which serves as the U.S. hub for Samsung's d...
Read More...
Jan 25, 2024
FDA Breakthrough Device Designation to Pi-Cardia’s ShortCut; AbSolutions Med’s REBUILD Bioabsorbable Abdominal Wall Closure Device; AngioDynamics Announces FDA 510(k) Clearance of Auryon XL Radial Access Catheter; Enhatch Announces FDA Clearance for a TKA Patient-Specific Instrumentation System
Pi-Cardia Receives FDA Breakthrough Device Designation for ShortCut™ Pi-Cardia Ltd., a prominent player in advancing catheter-based leaflet modification solutions for heart valve treatment, revealed that its ShortCut™ device has attained Breakthrough Device Designation from the US Food and Drug Administration. S...
Read More...
Jan 18, 2024
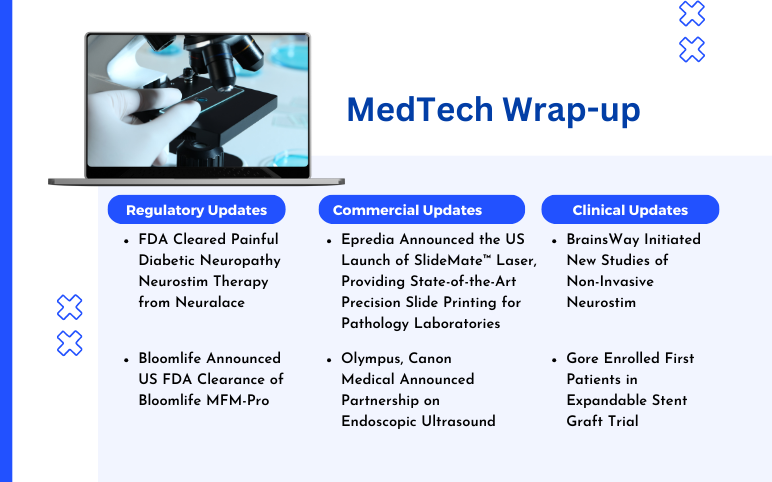
Epredia Announced the US Launch of SlideMate Laser; Olympus, Canon Medical Announced Partnership; FDA Cleared Painful Diabetic Neuropathy Neurostim Therapy from Neuralace; FDA Clearance to Bloomlife’s Bloomlife MFM-Pro; BrainsWay Initiated New Studies of Non-Invasive Neurostim; Gore Enrolled First Patients in Expandable Stent Graft Trial
Epredia Announced the US Launch of SlideMate™ Laser, Providing State-of-the-Art Precision Slide Printing for Pathology Laboratories On January 11, 2024, Epredia, a global leader in precision cancer diagnostics, announced that the company launched US sales of SlideMate™ Laser, the newest addition to the company’s...
Read More...
Jan 11, 2024
Medtronic Announced World’s First Approval for MiniMed 780G System; CE Mark for Medtronic’s Next Generation Micra Leadless Pacing Systems; Biosense Webster Received Approval for VARIPULSE PFA Platform; Rivermark Medical Updated on Flostent System; Cala Disclosed TAPS Therapy Data for Essential Tremor; Medline Introduced Transparent Wound Dressing
Medtronic Received CE Mark for its Next Generation Micra Leadless Pacing Systems On January 5, 2024, Medtronic announced that the Micra AV2 and Micra VR2, the next generation of its industry-leading tiny, leadless pacemakers, earned the CE (Conformité Européenne) Mark. The world's tiniest pacemakers, the rMic...
Read More...
Jan 04, 2024
HR Pharmaceuticals Announced Collaboration with Poiesis Medical; Enovis Acquired Lima Corporate; AnX Robotica Announced FDA Clearance for ProScan; FDA Breakthrough Device Designation for CGBio’s ‘NOVOSIS PUTTY’; EndoSound Received 510(k) Clearance for Breakthrough EVS Innovation; Boston Scientific Initiated AVANT GUARD Clinical Trial
HR Pharmaceuticals, Inc. Received Exclusive Commercialization Rights to Poiesis Medical’s Dual Balloon Catheter Technology in North America On December 28, 2023, HR Pharmaceuticals entered into a collaboration with Poiesis Medical LLC, to license Poiesis's Dual Balloon Catheter (Duette™). According to the terms ...
Read More...





-Agonist.png)

