MedTech News
Dec 28, 2023
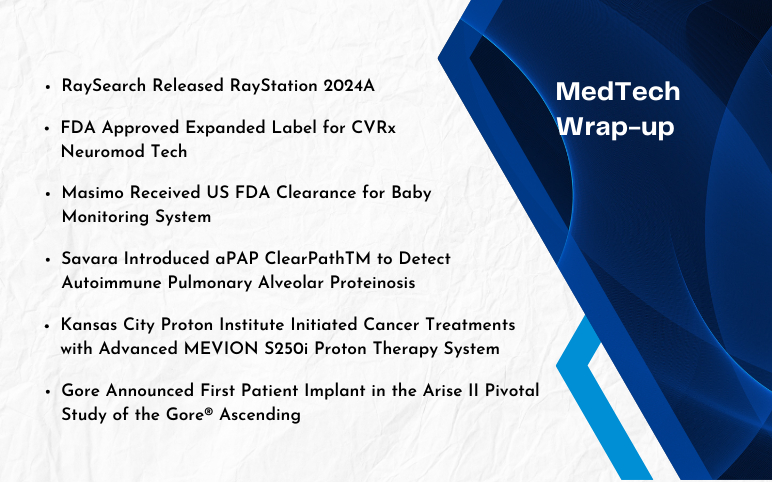
Savara Introduced aPAP ClearPathTM; RaySearch Released RayStation 2024A; FDA Approved Expanded Label for CVRx Neuromod Tech; FDA Clearance to Masimo’s Baby Monitoring System; Kansas City Proton Institute’s Advanced MEVION S250i Proton Therapy System; Gore’s Arise II Pivotal Study of the Gore® Ascending Stent Graft
Savara Introduced aPAP ClearPathTM to detect Autoimmune Pulmonary Alveolar Proteinosis On December 21, 2023, Savara, a clinical stage biopharmaceutical company launched aPAP ClearPathTM, which is used by the physicians to obtain a conclusive diagnosis of Autoimmune Pulmonary Alveolar Proteinosis (aPAP), a ...
Read More...
Dec 07, 2023
Johnson & Johnson Medtech Acquired Laminar; BD Launched Advanced Vascular Access Ultrasound System; FDA Cleared Oral Device for Severe Sleep Apnea; FDA Clearanced the Zilia OcularTM FC Retinal Camera; First Patient Enrolled in Penumbra Study of Computer-assisted Vacuum Thrombectomy; US FDA Granted the BiVACOR Total Artificial Heart IDE Approval for First-in-Human Early Feasibility Study
FDA Cleared Oral Device for Severe Sleep Apnea From Vivos Therapeutics On November 29, 2023, Vivos Therapeutics announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Vivos’s removable CARE (Complete Airway Repositioning and Expansion) oral appliances developed for treat...
Read More...
Nov 23, 2023
Ethicon’s ETHIZIA Hemostatic Sealing Patch; FDA Approves Medtronic’s Minimally Invasive Device to Treat Hypertension; Boston Scientific Acquires Relievant Medsystems; AstraZeneca Launched Evinova; Surmodics Announced Data of the SWING Trial; GSK Advancing the Low Carbon Ventolin Program to Phase III Trials
Ethicon Announced European Approval and Introduction of ETHIZIA™ Hemostatic Sealing Patch Used to Stop Disruptive Bleeding On November 15, 2023, Ethicon, a Johnson & Johnson MedTech company, received approval for ETHIZIA™, an adjunctive hemostat solution that has been clinically proven to achieve sustained h...
Read More...
Nov 09, 2023
SIBIONICS’s Ground-breaking GS1 Continuous Glucose Monitoring System; Abbott’s HPV Test to Run on Alinity m; CleanNA’s CE-IVD Molecular Diagnostics Product; Orthofix’s WaveForm L Interbody System; Alucent Biomedical’s AlucentNVS Technology; Surmodics’s TRANSCEND Trial Data
SIBIONICS Received CE Mark for Its Ground-breaking GS1 Continuous Glucose Monitoring System On November 01, 2023, SIBIONICS, the world's third-largest Continuous Glucose Monitoring System (CGM) brand, received the CE Mark for its revolutionary GS1 CGM. This significant milestone marks a momentous achiev...
Read More...
Nov 02, 2023
Laborie Medical Acquired Urotronic; Sirtex’s LAVA Liquid Embolic System; Paige’s Cancer Detection in Breast Lymph Nodes; FDA Clearance to Neurovalens’s Non-Invasive Insomnia-Treating Device; Recor Medical’s Renal Denervation System; Pulsecare Medical’s nsPFA Clinical Trial Short-term Follow-up Results
Neurovalens Received US FDA Clearance for Non-Invasive Insomnia-Treating Device On October 30, 2023, Belfast, Northern Ireland-based Neurovalens announced the US Food and Drug Administration clearance for its Modius Sleep device meant for treating chronic insomnia. Modius has been designed to deli...
Read More...
Oct 26, 2023
Cardio Flow’s FreedomFlow Orbital Atherectomy Peripheral Platform; Medtronic’s Aurora EV- ICD System to Treat Arrhythmias; GE Healthcare and Novo Nordisk Announces Collaboration; Olympus’s EVIS X1 Endoscopy System; Medtronic’s Evolut TAVR Platform Outperformed Surgery with Sustained Valve Performance; Abbott’s Minimally Invasive Devices for People with Leaky Heart Valves
Olympus Announced Market Launch of EVIS X1™ Endoscopy System On October 19, 2023, Olympus Corporation, a global medical technology company announced the market launch of its next-generation EVIS X1™ endoscopy system. The GIF-1100 gastrointestinal videoscope indicated for use in the upper digestive tract, and ...
Read More...
Oct 05, 2023
Boston Scientific’s Insertable Cardiac Monitor; Phillips-Medisize Teamed up with GlucoModicum; Boston Scientific’s Intravascular Ultrasound System; Enable Injections Received First US FDA Approval; Amber Implants’s VCFix® Spinal System; Loci Orthopaedics’s “InDx” Thumb Base Joint Replacement Study
Boston Scientific Launched Next-Generation Insertable Cardiac Monitor On October 2, 2023, Boston Scientific, announced the launch of its next-generation LUX-Dx II+ insertable cardiac monitor (ICM) system. Long-term monitoring is provided by the system for arrhythmias connected to conditions like AFib, c...
Read More...
Sep 21, 2023
Zepp Health Launched OTC Hearing Aids; Neoss Launched NeoScan 2000; FDA Clearance for AI-Assisted Sleep Monitoring Device Dreem 3S; Tasso Received CE Mark Certification for Tasso+; Neuralink’s First-in-human BCI trial; Creative Medical to Initiate a Phase I/II Clinical Trial of StemSpine
Zepp Health Launched a New Line of OTC Hearing Aids for its Zepp Clarity Brand On September 19, 2023, Zepp Clarity, a smart hearing solutions brand owned by Zepp Health, a health technology company, announced the launch of Zepp Clarity Pixie, a next-generation premium hearing solution. The Pixie, which ...
Read More...
Sep 07, 2023
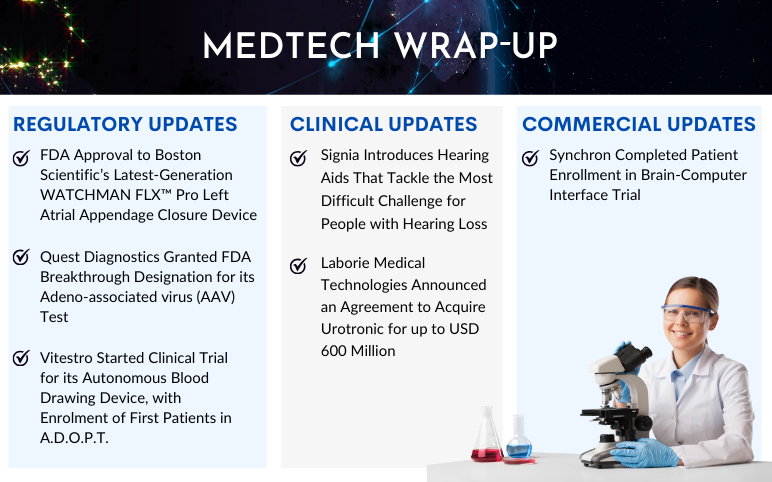
Boston Scientific’s WATCHMAN FLX™ Pro; Quest Diagnostics’s AAV Test; Vitestro Started A.D.O.P.T. Clinical Trial; Signia Introduces Hearing Aids; Laborie Medical to Acquire Urotronic; Synchron’s Brain-Computer Interface Trial
Quest Diagnostics Granted FDA Breakthrough Designation for its Adeno-associated virus (AAV) Test On August 30, 2023, Quest Diagnostics announced that its AAVrh74 ELISA assay (CDx) has been granted Breakthrough Device Designation from the US Food and Drug Administration (FDA). The enzyme-linked immunosor...
Read More...
Aug 31, 2023
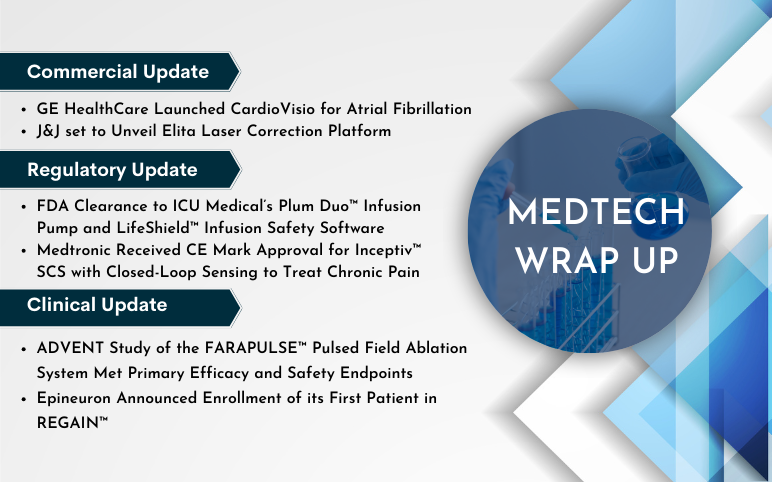
GE HealthCare Launched CardioVisio; J&J’s Elita Laser Correction Platform; ICU Medical’s Plum Duo Infusion Pump and LifeShield Infusion Safety Software Update; Medtronic’s Inceptiv SCS with Closed-Loop Sensing to Treat Chronic Pain; Boston Scientific’s FARAPULSE Pulsed Field Ablation System; Epineuron’s REGAIN Trial
GE HealthCare Launched CardioVisio for Atrial Fibrillation, a Digital, Patient-Centric Clinical Decision Support Tool to Enable Precision Care On August 24, 2023, GE HealthCare launched CardioVisio for Atrial Fibrillation (AFib), a digital tool designed to assist clinicians in visualizing longitudinal data relev...
Read More...






-Agonist.png)

