MedTech
Dec 30, 2021
Cerner, Scanwell Health and Personal Genome Diagnostics Acquisition; Zynex’s Fluid Monitoring System, CM-1500; Sight Sciences’ TearCare System; Envista’s Nobel Biocare N1 Implant System
Oracle Corporation sets to acquire Cerner Corporation On December 20, 2021, Oracle Corporation and Cerner Corporation entered into an agreement as per which Oracle will acquire Cerner through an all-cash tender offer for approximately USD 28.3 billion in equity value. Larry Ellison, Chairman and C...
Read More...
Dec 16, 2021
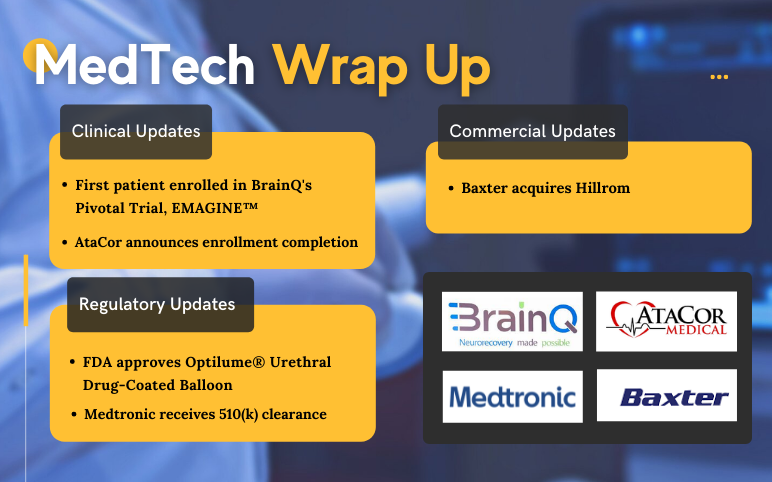
BrainQ’s EMAGINE Trial; AtaCor’s EV-ICD study; Urotronic’s Optilume Urethral Drug-Coated Balloon approval; Medtronic receives 510(k) clearance; Baxter acquires Hillrom
First patient enrolled in BrainQ's Pivotal Trial, EMAGINE™, for frequency-tuned electromagnetic field therapy to reduce disability following Ischemic Stroke On December 09, 2021, BrainQ announced the enrollment of the first patient at MedStar National Rehabilitation Hospital in its pivotal, randomized, ...
Read More...
Nov 25, 2021
Neurent Medical’s NEUROMARK; Brain Navi NaoTrac received European CE Mark approval; Theradaptive receives Breakthrough Medical Device Designation; HAGAR GWave awarded Breakthrough Device Designation; Fujifilm launches ColoAssist PRO
Neurent Medical obtains FDA Clearance for NEUROMARK™, a novel multi-point Nerve Disruption Treatment for Chronic Rhinitis On November 18, 2021, Neurent Medical, a company working in developing treatments for chronic inflammatory sino-nasal diseases, received clearance from the Food and Drug Ad...
Read More...
Oct 28, 2021
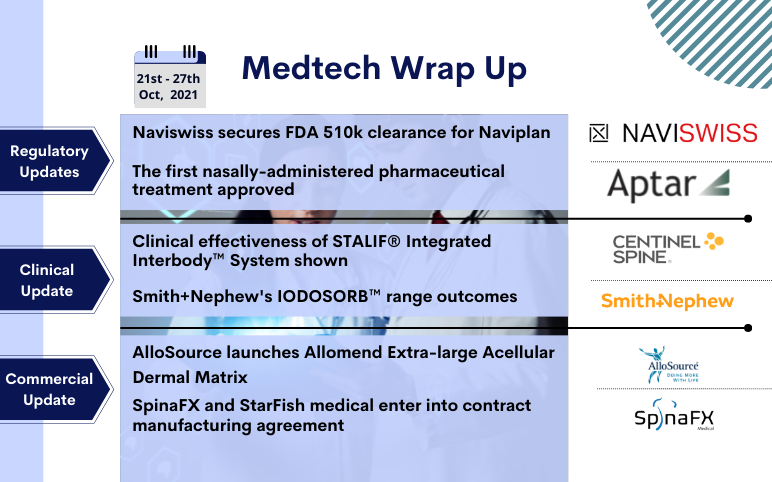
FDA 510k clearance to Naviswiss’s Naviplan; FDA approved to TYRVAYA; Centinel Spine’s STALIF Integrated Interbody System; Smith+Nephew’s IODOSORB; AlloSource’s AlloMend Extra-Large ADM; SpinaFX and StarFish’s strategic commercialisation contract
Naviswiss secures FDA 510k clearance for Naviplan, an advanced planning solution for hip replacement surgery On October 21, 2021, Naviswiss, a Swiss-based medical technology company, received approval from the FDA to commercialise Naviplan in the United States. Naviplan is a digital pre-operative planning ...
Read More...
Sep 02, 2021
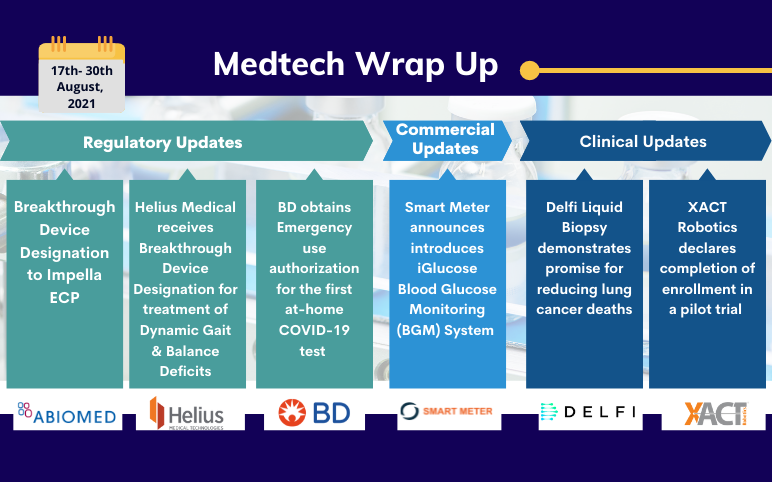
Abiomed’s Impella ECP; Helius’s PoNS Device; Smart Meter’s iGlucose BGM; XACT’s CT-Guided Percutaneous Procedures; BD’s Veritor At-Home COVID-19 Test; Delfi’s Liquid Biopsy
FDA awards Breakthrough Device Designation to Impella ECP On August 18, 2021, Abiomed received breakthrough device designation from the United States Food and Drug Administration (FDA) for its Impella ECP expandable percutaneous heart pump, the world’s smallest heart pump and the first to be compatible wit...
Read More...
Aug 19, 2021
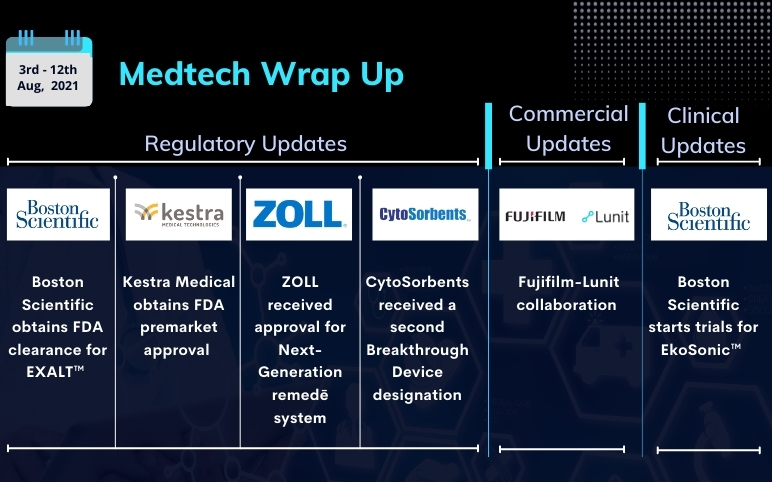
Boston Scientific’s EkoSonic; FDA clearance for EXALT; Kestra Medical’s ASSURE; ZOLL Next-Generation remedē system; CytoSorbents’ DrugSorb-ATR; Fujifilm-Lunit collaboration
Boston Scientific starts recruitment for randomized controlled trials for the EkoSonic™ Endovascular System On August 04, 2021, Boston Scientific Corporation started the enrolment in the HI-PEITHO clinical trial, which is a collaborative research study with the Pulmonary Embolism Response Team (PERT) Cons...
Read More...
Jun 03, 2021
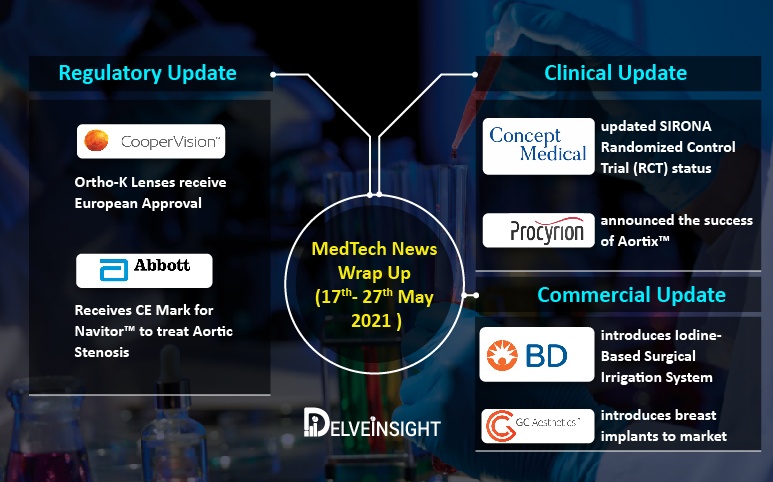
Latest Medtech Commercial, Regulatory and Clinical Updates For – Abbott, BD, CooperVision, Procyrion, TricValve, and others
Abbott receives CE Mark for Navitor™, the Latest-Generation Transcatheter Aortic Valve Implantation (TAVI) System for Aortic Stenosis treatment On May 17, 2021, Abbott received CE Mark for its latest-generation transcatheter aortic valve implantation (TAVI) system, Navitor™. This marks the minimal...
Read More...
May 20, 2021
MedTech Industry: Commercial, Regulatory and Clinical Updates
HCmed Signs a Global Development Agreement with CSL Behring to develop a combination product On May 04, 2021, HCmed has announced the start of a strategic partnership with global biotherapeutics leader CSL Behring to collaboratively develop CSL Behring's plasma-derived immunoglobulin administered via HCmed’s new...
Read More...
May 04, 2021
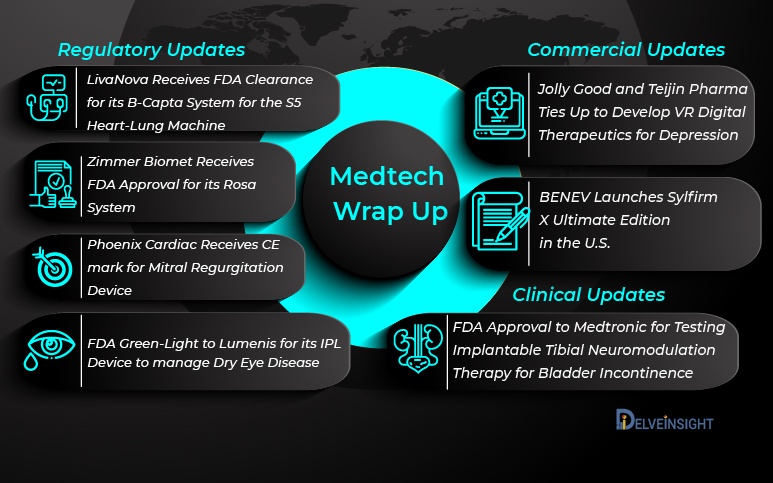
MedTech Industry Roars Back as FDA Approvals Soar
Zimmer Biomet Receives FDA Approval for its Rosa System On 20 April 2021, Zimmer Biomet received FDA 510(k) clearance for its Rosa Partial Knee system for robotically-assisted partial knee replacement surgeries. This is the new edition to Zimmer Biomet's Rosa Robotics platform, which comprises the Rosa Knee syst...
Read More...
Dec 07, 2020
Creating Future of MedTech Industry with Artificial Intelligence
Artificial Intelligence is a breakthrough technology and has been used tremendously in this data-driven age, leading to a leap forward in the MedTech industry. Artificial Intelligence enrolls the use of advanced machine learning algorithms and fulfills the job, which has been tackled by humans for ages. It has flou...
Read More...




-Agonist.png)

