Ophthalmic Devices Market
Aug 06, 2025
From Sight to Insight: The Rise of Smart Ophthalmological Devices in Eye Care
Ophthalmology devices are specialized medical instruments and equipment designed for the diagnosis, treatment, and management of eye-related conditions and disorders. These devices encompass a wide range of technologies, including diagnostic tools like optical coherence tomography (OCT) scanners and fundus cameras,...
Read More...
Mar 28, 2024
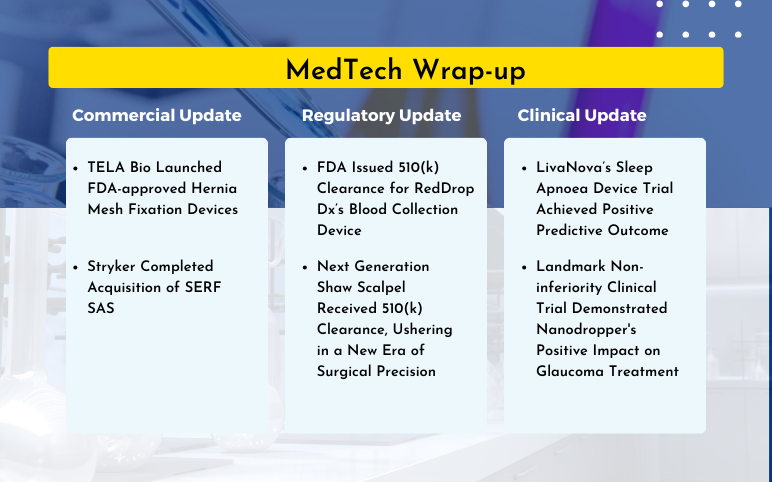
TELA Bio Launched Hernia Mesh Fixation Devices; Stryker Acquisition of SERF SAS; FDA 510(k) Clearance for RedDrop Dx’s Blood Collection Device; FDA 510(k) Clearance for Next Generation Shaw Scalpel; LivaNova’s Sleep Apnoea Device Trial; Nanodropper’s Positive Impact on Glaucoma Treatment
TELA Bio Launched FDA-approved Hernia Mesh Fixation Devices On March 21, 2024, TELA Bio, Inc., a medical technology firm, launched its FDA-approved LIQUIFIX devices for hernia repairs in the United States. The LIQUIFIX FIX8™ and LIQUIFIX Precision™ are specifically engineered for laparoscopic and open femo...
Read More...
Dec 07, 2023
Johnson & Johnson Medtech Acquired Laminar; BD Launched Advanced Vascular Access Ultrasound System; FDA Cleared Oral Device for Severe Sleep Apnea; FDA Clearanced the Zilia OcularTM FC Retinal Camera; First Patient Enrolled in Penumbra Study of Computer-assisted Vacuum Thrombectomy; US FDA Granted the BiVACOR Total Artificial Heart IDE Approval for First-in-Human Early Feasibility Study
FDA Cleared Oral Device for Severe Sleep Apnea From Vivos Therapeutics On November 29, 2023, Vivos Therapeutics announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Vivos’s removable CARE (Complete Airway Repositioning and Expansion) oral appliances developed for treat...
Read More...
Jun 30, 2022
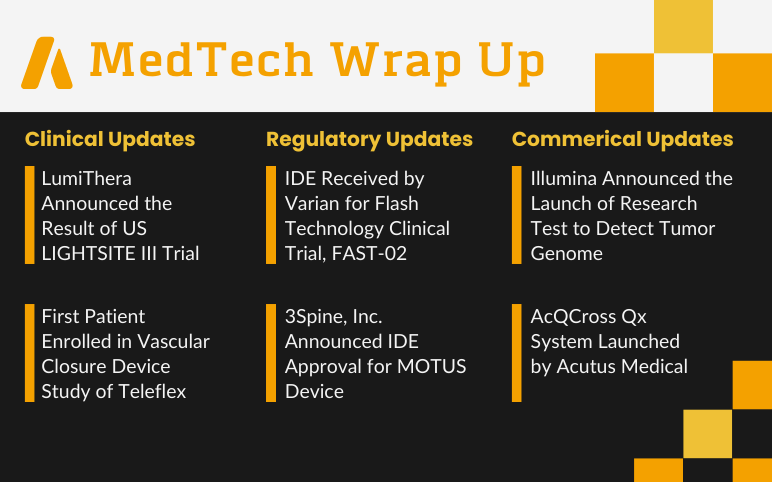
LumiThera’s US LIGHTSITE III Trial; First Patient Enrolled in Vascular Closure Device Study of Teleflex; Varian’s Flash Technology Clinical Trial, FAST-02; 3Spine’s MOTUS Device; Illumina’s Research Test to Detect Tumor Genome; Acutus Medical’s AcQCross Qx System
LumiThera Announced the Result of US LIGHTSITE III Trial of Non-neovascular Age-Related Macular Degeneration (AMD) Subjects Treated with Photobiomodulation (PBM) using the Valeda® Light Delivery System On June 22, 2022, LumiThera Inc., a commercial-stage medical device company offering photobiomodulation (PBM) t...
Read More...
Apr 14, 2022
Alcon’s Dry Eye Treatment Device; I-VASC Secures Series A Funding; Paige’s AI Medical Device Software; TRUVIC’s Prodigy Thrombectomy System; Alucent’s Natural Vascular Scaffolding Technology; Guardant Presents Data Supporting Efficacy of Blood Test in Detecting Multiple Cancers
Alcon Announces Launch of Dry Eye Treatment Device On April 04, 2022, Alcon Inc. announced the launch of their latest dry eye innovation, the Systane® iLux2® Meibomian Gland Dysfunction (MGD) Thermal Pulsation System. It is an all-in-one handheld device equipped with new imaging technology to capture infrared ph...
Read More...


-Agonist.png)

