Pain Management Devices
Jul 03, 2025
FDA Clears Stryker’s Incompass Total Ankle System; Glaukos Receives EU MDR Approval for iStent infinite and Key MIGS Devices; STENTiT Announces First Patient Enrollment in Trial of Regenerative Stent for Limb Preservation; Autonomix Medical Treats First Patient in Next Phase (“PoC 2”) of Proof-of-Concept Clinical Trial; Getinge Expands Servo-C Ventilator Capabilities for Neonatal Care; Medtronic Partners With Philips to Advance Patient Monitoring Solutions
Stryker Received FDA Clearance for Incompass Total Ankle System On June 25, 2025, Stryker, a global leader in medical technologies, announced that the U.S. Food and Drug Administration (FDA) received 510(k) approval for its Incompass™ Total Ankle System. The implant is designed for patients suffering from ...
Read More...
Jan 23, 2025
Tempus Launches FDA-Approved xT CDx Test Nationwide; B. Braun Enhances Catheter Securement with the Launch of Clik-FIX; Illuccix® Secures European Regulatory Approval; FDA Clears CapsoCam Plus® for Remote Ingestion; BellaSeno Marks Success in Two Clinical Trials for Revolutionary Breast Implant Technology; AF Symposium to Feature Conformal Medical’s Groundbreaking GLACE Study
Tempus Announced the National Launch of the FDA-Approved xT CDx Test On January 15, 2025, Tempus AI, Inc., a technology company driving the use of AI to advance precision medicine and improve patient care, announced the nationwide launch of its FDA-approved, NGS-based in vitro diagnostic device, xT CDx. Th...
Read More...
Jul 11, 2024
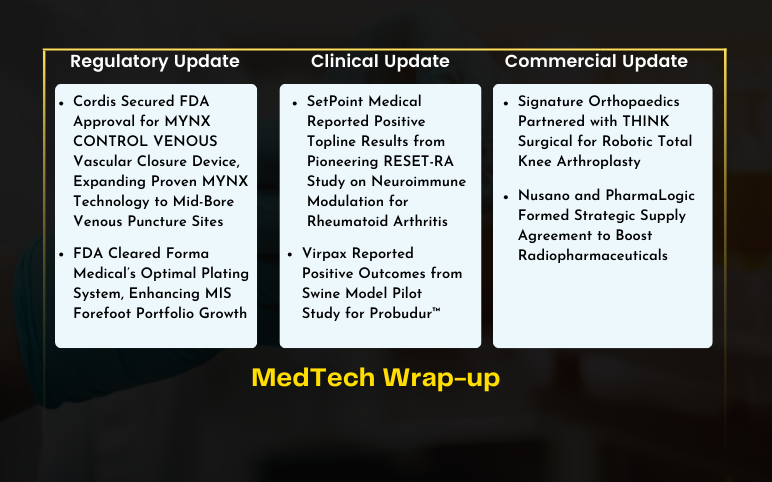
Cordis FDA Approval for MYNX CONTROL VENOUS Vascular Closure Device; Forma Medical’s Optimal Plating System FDA Approval; Virpax’s Swine Model Pilot Study Positive Results; SetPoint Medical Positive Topline Results from Pioneering RESET-RA Study; Signature Orthopaedics Partnered with THINK Surgical; Nusano and PharmaLogic Formed Strategic Supply Agreement
Cordis Secured FDA Approval for MYNX CONTROL VENOUS Vascular Closure Device, Expanding Proven MYNX Technology to Mid-Bore Venous Puncture Sites On July 9, 2024, Cordis, a leading company in cardiovascular and endovascular technology, received FDA approval for its MYNX CONTROL™ VENOUS Vascular Closure Device. Thi...
Read More...
Jun 27, 2024
Alcon’s 510(k) Clearance From the FDA; Globus Medical Received FDA Clearance; UK Pioneers First Skull-Mounted Epilepsy Device for Child; The Initiation of a Significant Clinical Trial of 3d Printed Models; Esaote Unveiled the Cutting-Edge Mylab™E80 @Sirm Ultrasound Device For 2024; Medasense Unveiled a Transformative Partnership With Nihon Kohden Corporation
Alcon's Newest Technological Marvels, Unity VCS, and Unity CS, Achieved 510(k) Clearance From the U.S. FDA, Paving the Way for New Advancements On June 24, 2024, Alcon, the foremost name in eye care with a mission to help people see brilliantly, revealed that the U.S. Food and Drug Administration (FDA) had...
Read More...
May 16, 2024
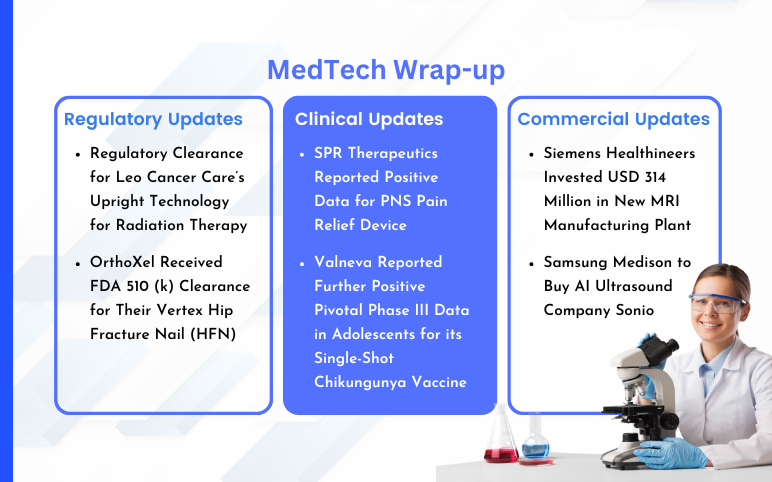
Siemens Healthineers USD 314 Million Investment; Samsung Medison’s Sonio Acquisition; Leo Cancer Care’s Upright Technology Clearance; OrthoXel’s Vertex Hip Fracture Nail FDA 510 (k) Clearance; SPR Therapeutics’ PNS Pain Relief Device Positive Data; Valneva’s Chikungunya Vaccine Positive Pivotal Phase III Data
Siemens Healthineers Invested USD 314 Million in New MRI Manufacturing Plant On May 15, 2024, Siemens Healthineers announced that it had invested USD 314 million in a new MRI manufacturing plant in the UK. The facility in Oxford, England, which Siemens anticipates opening in 2026, will develop technology to redu...
Read More...



-Agonist.png)

