Stents
Jul 31, 2025
Understanding How Cardiac Devices are Aiding in Treatment and Prevention of Heart Disease
Cardiovascular diseases (CVDs) continue to dominate as the leading global cause of death, responsible for approximately 17.9 million deaths annually, as reported by the World Health Organization (2021). Representing the highest disease burden worldwide, CVD incidence is rising rapidly across most low- and middle-in...
Read More...
Jun 20, 2024
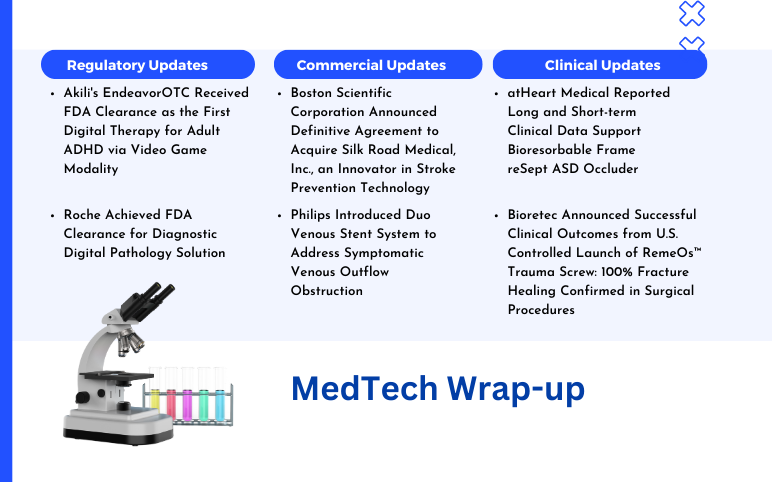
Boston Scientific Corporation Agreement to Acquire Silk Road Medical; Philips Introduced Duo Venous Stent System; Akili’s EndeavorOTC Received FDA Clearance; Roche Achieved FDA Clearance; atHeart Medical Reported Long and Short-term Clinical Data; Bioretec Announced Successful Clinical Outcomes
Boston Scientific Corporation Announced Definitive Agreement to Acquire Silk Road Medical, Inc., an Innovator in Stroke Prevention Technology On June 18, 2024, Boston Scientific Corporation, a global leader in medical technologies, announced the formal agreement to acquire Silk Medical, Inc., a medical device co...
Read More...
Dec 28, 2023
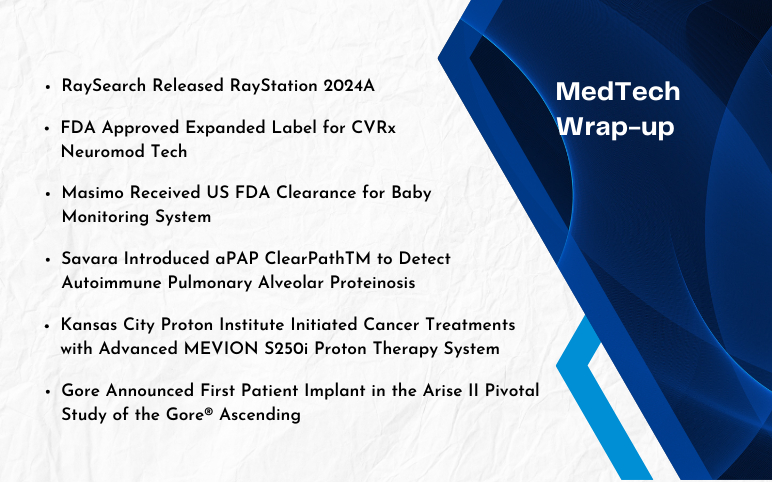
Savara Introduced aPAP ClearPathTM; RaySearch Released RayStation 2024A; FDA Approved Expanded Label for CVRx Neuromod Tech; FDA Clearance to Masimo’s Baby Monitoring System; Kansas City Proton Institute’s Advanced MEVION S250i Proton Therapy System; Gore’s Arise II Pivotal Study of the Gore® Ascending Stent Graft
Savara Introduced aPAP ClearPathTM to detect Autoimmune Pulmonary Alveolar Proteinosis On December 21, 2023, Savara, a clinical stage biopharmaceutical company launched aPAP ClearPathTM, which is used by the physicians to obtain a conclusive diagnosis of Autoimmune Pulmonary Alveolar Proteinosis (aPAP), a ...
Read More...
Oct 12, 2023
B. Braun’s Introcan Safety 2 IV Catheter; Everly Health’s At-Home Collection Kidney Health Test; FDA Clearance to Sanguina’s AnemoCheck Home; FDA Clearance for the DePuy’s TriALTIS™ Spine System; Biotronik’s Spinal Cord Stimulation Tech; Contego Medical’s Performance III Direct Transcarotid Access Stenting Trial
B. Braun Launched Introcan Safety® 2 IV Catheter with Multi-Access Blood Control Designed to Protect Clinicians Every Time the Hub is Accessed On October 11, 2023, B. Braun Medical Inc. (B. Braun), a leader in smart infusion therapy, announced the launch of its new Introcan Safety® 2 IV Catheter with Multi-Acces...
Read More...
Jun 15, 2023
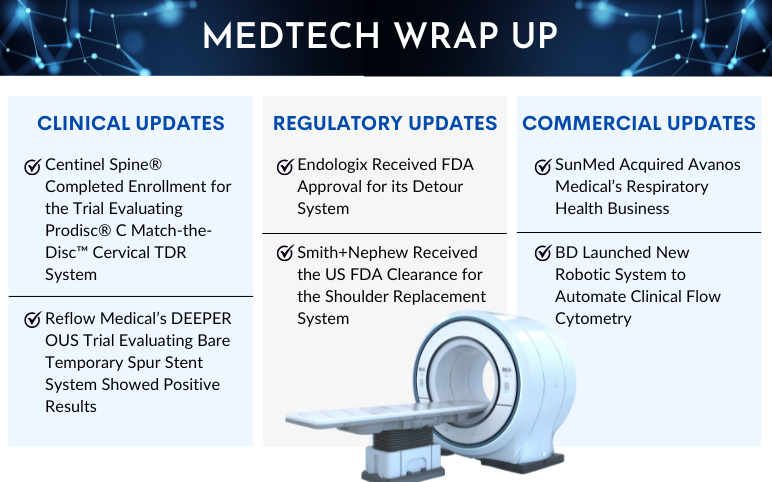
Centinel Spine’s Prodisc C Match-the-Disc Cervical TDR System; Reflow Medical’s DEEPER OUS Trial; FDA Approval to Endologix’s Detour System; FDA Clearance to Smith+Nephew’s Shoulder Replacement System; SunMed Acquired Avanos’s Respiratory Health Business; BD’s Automate Clinical Flow Cytometry
Centinel Spine® Completed Enrollment in First-of-its-Kind 2-Level IDE Trial Evaluating prodisc® C Match-the-Disc™ Cervical TDR System On June 13, 2023, Centinel Spine®, LLC, a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-pr...
Read More...
Jul 07, 2022
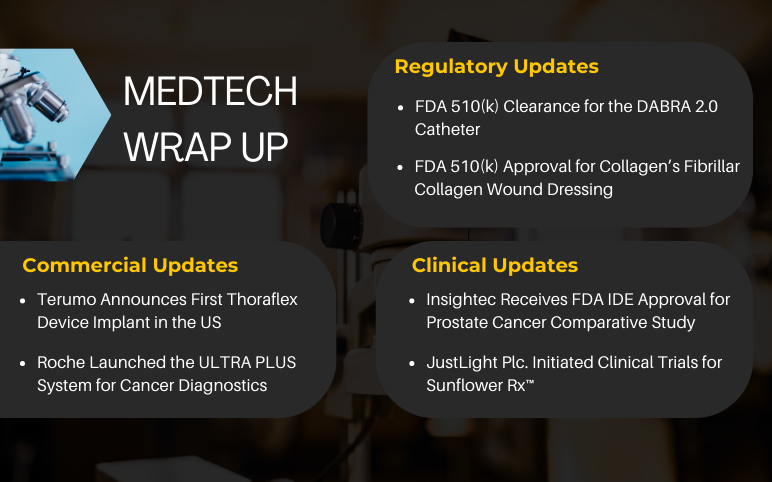
Collagen Matrix FDA 510(k) approval for Fibrillar Collagen Wound Dressing; Roche’s cancer diagnostics ULTRA PLUS system launch; Insightec IDE Approval for Prostate Cancer; JustLight plc. trial of Sunflower Rx for Alzheimer’s disease; Thoraflex Hybrid Device Implantation in the United States; FDA 510(k) Clearance for the DABRA 2.0 Catheter
Collagen Matrix received FDA 510(k) approval for Fibrillar Collagen Wound Dressing On June 29, 2022, Collagen Matrix, Inc., a leader in regenerative medicine, a global manufacturer of collagen and other biomaterial-based medical devices, and Linden Capital Partners portfolio company announced FDA 510(k) Clearan...
Read More...

-Agonist.png)

