Surgical Robotic System Market
Nov 27, 2025
Bracco Receives FDA Clearance for Additional Clinical Use of Max 3™ Syringeless MR Injector; RapidAI Broadens Clinical AI Portfolio with Five Newly Granted FDA Clearances; Ceribell Secures FDA 510(k) Clearance for Clarity® Algorithm in Neonatal Care; BD Introduces Surgiphor™ Irrigation System Across Europe to Enhance Surgical Patient Protection; Neurophet and The Florey Announce Strengthened Collaboration to Advance Alzheimer’s Diagnostic Solutions; VSI® Performs First-Ever Robotic Minimally Invasive Bertolotti’s Resection; BioWave® Demonstrates Safe and Effective Home-Based and War Injury Pain Relief in Latest Clinical Data
FDA Approved Expanded Indication for Max 3™ Syringeless MR Injector from Bracco On November 25, 2025, Bracco Diagnostics Inc., the U.S. subsidiary of Bracco Imaging S.p.A., announced that the U.S. Food and Drug Administration (FDA) expanded the indication for its Max 3™ Rapid Exchange and Syringeless Injector fo...
Read More...
Oct 09, 2025
Vektor Medical Receives CE Mark for vMap; Tosoh’s GR01 HbA1c Analyzer Gains FDA 510(k) Clearance; Zimmer Biomet Acquires Monogram Technologies; Olympus Partners with W. L. Gore & Associates to Distribute GORE® VIABIL® Biliary Stent Globally; Medtronic Launches U.S. IDE Study on Hugo™ Robotic Surgery System for Gynecology; Envoy Medical Secures FDA Clearance to Advance Pivotal Trial to Final Stage Following Promising Three-Month Results
Vektor Medical Secures CE Mark for vMap, Bringing the Benefits of Non-Invasive Arrhythmia Mapping to Europe On October 7, 2025, Vektor Medical, a leading innovator in cardiac arrhythmia care, received CE Mark approval for its vMap® System, a non-invasive, AI-powered technology that converts standard 12-lea...
Read More...
Sep 25, 2025
Medtronic Gains FDA Clearance for Altaviva™ Device to Simplify Treatment of Urge Urinary Incontinence; FDA Grants Breakthrough Device Status to Virtuoso’s Surgical Robot; Lunit and Agilent Strike Partnership to Integrate AI into Biomarker-Based Diagnostics; QuidelOrtho Launches QUICKVUE Influenza + SARS Test for Clinical Use; Transpara® Breast AI Chosen to Power $16M PRISM Clinical Trial in the U.S.; IceCure Showcases ProSense® at CIRSE 2025 with Strong Clinical Evidence from 4 Independent Cryoablation Studies
Medtronic Secured FDA Approval for the Altaviva™ Device, Delivering a Simpler Experience in Treating Urge Urinary Incontinence On September 19, 2025, Medtronic plc, a global leader in healthcare technology, announced that it had received U.S. Food and Drug Administration (FDA) approval for the Altaviva™ de...
Read More...
Sep 11, 2025
ONWARD Medical’s ARC-EX System Gains CE Mark; Microbot Medical Secures FDA 510(k) Clearance for LIBERTY® Endovascular Robotic System; Fraiya Launches Major NHS Clinical Trial Using AI for Pregnancy Ultrasounds; Medtronic Hugo™ Robotic-assisted Surgery System in Hernia Repair Met Safety and Effectiveness Endpoints; Willow Launches Willow Wave, Pioneering a New Standard in Breastfeeding; Vivalink Launches New Adhesive to Improve Wearable ECG Performance
ONWARD Medical Received CE Mark for ARC-EX, Enabling Commercial Launch of Breakthrough Spinal Cord Stimulation System in Europe On September 8, 2025, ONWARD Medical N.V., a leading neurotechnology company developing therapies to restore movement, function, and independence for individuals with spinal cord ...
Read More...
Jul 17, 2025
Medtronic Gains CE Mark for LigaSure™ on Hugo™ Robotic System; J&J Wins FDA Nod for VARIPULSE™ Irrigation Update; DSMB Clears Continuation of Aethlon Medical Clinical Study; MicroPort® CardioFlow Marks First VitaFlow Liberty® Implants in South Korea; Olympus Unveils EU-ME3 Ultrasound Processor in U.S.; QuidelOrtho, BÜHLMANN Launch fCAL® & fPELA® Turbo Assays on VITROS™
Medtronic Secured CE Mark for LigaSure™ Technology Integration on Hugo™ Robotic-Assisted Surgery System, Paving the Way for the Future of Surgery On July 15, 2025, Medtronic plc, a global leader in healthcare technology, announced that it received CE Mark approval for its LigaSure™ RAS vessel-sealing techn...
Read More...
Jul 10, 2025
Intuitive’s da Vinci 5 Gets CE Mark; Boston Scientific’s FARAPULSE™ Secures Expanded FDA Approval; Trividia Health’s TRUE METRIX® Added to Pennsylvania Medicaid List; Cerapedics’ PearlMatrix™ Sees First U.S. Clinical Use Post-FDA Nod; Smith+Nephew Expands Q-FIX◊ with Knotless Option; Cochlear Unveils World’s First Smart Cochlear Implant
Intuitive’s da Vinci 5 Surgical System Received CE Mark On July 02, 2025, Intuitive, widely recognized as a pioneer in robotic-assisted surgery and minimally invasive care, announced that its latest system, the da Vinci 5, had secured CE mark approval for use in both adult and pediatric patients across Eur...
Read More...
Apr 17, 2025
Edwards’ SAPIEN M3 Becomes First Transfemoral TMVR System to Receive CE Mark; Terumo Neuro Receives FDA Clearance for Carotid Stent System; Microbot Medical Reports Complete Robotic Navigation Success in LIBERTY® System Pivotal Trial; J&J MedTech Marks Milestone with First OTTAVA™ Robotic Surgeries; Baxter Introduces Room-Temperature Hemopatch for Faster Surgical Hemostasis; Merit Medical Introduces the Ventrax™ Delivery System
Edwards' SAPIEN M3 Received CE Mark as the World’s First Transfemoral TMVR System On April 14, 2025, Edwards Lifesciences Corporation announced that its SAPIEN M3 mitral valve replacement system had received CE Mark for the transcatheter treatment of patients with symptomatic (moderate-to-severe or severe)...
Read More...
Dec 12, 2024
Stereotaxis and Shanghai MicroPort EP Medtech Co., Ltd. Receives Approval for Magbot Robotic Magnetic Navigation Ablation Catheter From China’s NMPA; AngioDynamics NanoKnife® System Approved by FDA for Prostate Tissue Treatment; Zynex Successfully Completes Laser Pulse Oximetry Clinical Trial; COTA, PreciseDx, and Baptist Health Validate AI-Powered PreciseBreast as Equivalent to Oncotype DX; Medtronic Introduces Percept™ RC Rechargeable Neurostimulator; Crown Aesthetics Raises the Bar with the Introduction of SkinPen® Precision Elite
China's NMPA Approved Magbot Robotic Magnetic Navigation Ablation Catheter On December 9, 2024, Stereotaxis and Shanghai MicroPort EP Medtech Co., Ltd., announced that the Magbot™ Magnetic Navigation Ablation Catheter received regulatory approval from China’s National Medical Products Administration (NMPA). T...
Read More...
Oct 17, 2024
Roche Receives FDA Approval for Itovebi; FDA Approves Bausch + Lomb’s Envista® Envy™; Microbot Medical Successfully Completes Pivotal Human Clinical Trial and Accelerates Go-to-Market Strategy for LIBERTY® Launch; Positive FDA Guidance on Phase III Trial of 64Cu-SAR-Bispsma for Recurrent Prostate Cancer; ELEHEAR Launches Hearing Aids at Just $399; Abbott Advances Pulsed Field Ablation Studies and Launches New Technology for Cardiac Mapping
FDA Approved Roche’s Itovebi, a Targeted Treatment for Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer With a PIK3CA Mutation On October 11, 2024, Roche announced that the United States Food and Drug Administration (FDA) approved Itovebi™ (inavolisib), in combination with palbociclib (Ibran...
Read More...
Sep 19, 2024
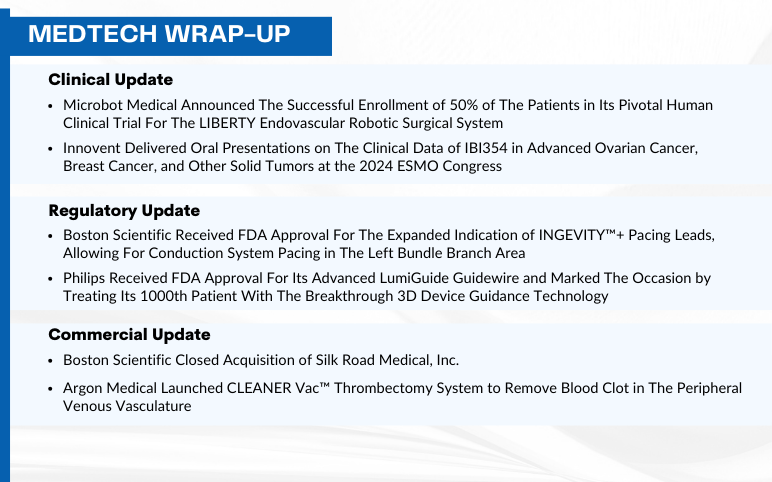
Boston Scientific INGEVITY™ gets Expanded Indication and acquires Silk Road Medical; FDA Approves Philips’ LumiGuide Guidewire; Microbot Completes LIBERTY Trial Enrollment; Innovent Presents Data at ESMO 2024; Argon Medical Launches CLEANER Vac™ System
Boston Scientific Received FDA Approval For The Expanded Indication of INGEVITY™+ Pacing Leads, Allowing For Conduction System Pacing in The Left Bundle Branch Area On September 17, 2024, Boston Scientific Corporation received U.S. Food and Drug Administration (FDA) approval to expand the indication for th...
Read More...







-Agonist.png)

