AXL Receptor Tyrosine Kinase Inhibitors Market
- According to DelveInsight, AXL Receptor Tyrosine Kinase Inhibitors Market is expected to grow at a decent CAGR by 2034
- AXL, a member of the TAM (Tyro3, Axl, Mer) family, and its inhibitors can specifically break the kinase signaling nodes, allowing advanced patients to regain drug sensitivity with improved therapeutic efficacy.
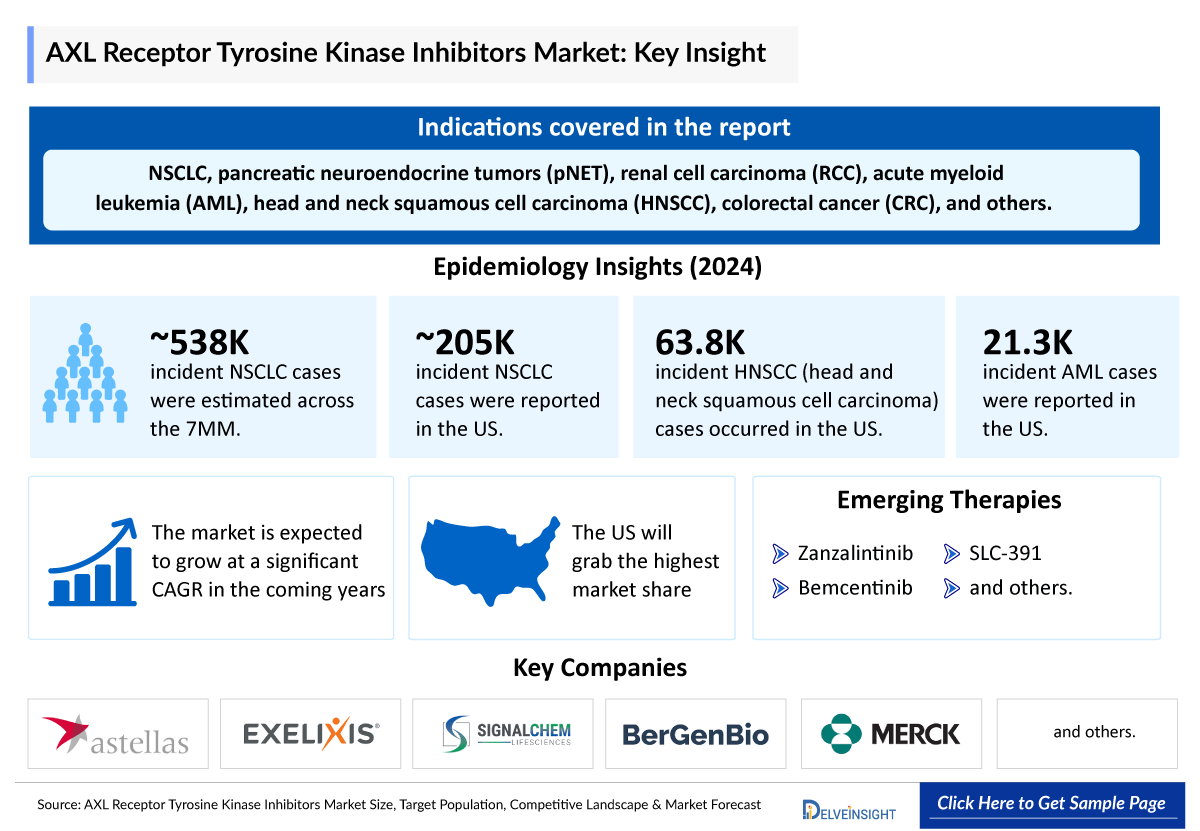
- AXL-targeted therapies are being explored across multiple cancer types, including non-small cell lung cancer (NSCLC), pancreatic neuroendocrine tumors (pNET), renal cell carcinoma (RCC), acute myeloid leukemia (AML), head and neck squamous cell carcinoma (HNSCC), colorectal cancer (CRC), and others.
- In March 2025, the US FDA approved CABOMETYX for adult and pediatric patients aged 12 years and older with previously treated, unresectable, locally advanced or metastatic, well-differentiated pNET and extra-pancreatic neuroendocrine tumors (epNET).
- The National Comprehensive Cancer Network (NCCN) has recognized the CABOMETYX-nivolumab combination as a Category 1 preferred first-line treatment for clear cell RCC across all risk groups and a Category 2A option for first-line non-clear cell RCC. Additionally, single-agent CABOMETYX is recommended for previously treated advanced clear cell RCC, reinforcing its role across multiple lines of RCC therapy.
- In January 2025, BergenBio announced that the first patient had been included in a clinical trial evaluating bemcentinib in lung cancer. The trial is sponsored by the Mays Cancer Center at The University of Texas Health Science Center in San Antonio. The Phase II portion of the trial will study the dose(s) selected in Phase Ib. It will assess the overall response rate and progression-free survival of treated patients.
- AXL receptor tyrosine kinase inhibitors, including CABOMETYX (cabozantinib), and others have received approval from regulatory bodies.
- BerGenBio, Signalchem Lifesciences, and several other companies are currently engaged in the development and production of selective AXL receptor TKI, which have the potential to significantly impact and enhance the AXL inhibitor market.
DelveInsight’s “AXL Receptor Tyrosine Kinase Inhibitors (TKI) Market Size, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the AXL receptor TKI, historical and Competitive Landscape as well as the AXL receptor TKI market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The AXL receptor TKI market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM AXL receptor TKI market size from 2020 to 2034. The report also covers current AXL receptor TKI treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the AXL Receptor Tyrosine Kinase Inhibitors market’s potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
AXL Receptor Tyrosine Kinase Inhibitors Epidemiology |
Segmented by:
|
|
AXL Receptor Tyrosine Kinase Inhibitors Companies |
|
|
AXL Receptor Tyrosine Kinase Inhibitors Therapies |
|
|
AXL Receptor Tyrosine Kinase Inhibitors Market |
Segmented by:
|
|
Analysis |
|
AXL Receptor Tyrosine Kinase Inhibitors Market: Understanding and Treatment Algorithm
AXL Receptor Tyrosine Kinase Inhibitors Overview
AXL receptor tyrosine kinase is a member of the tyrosine‑protein kinase receptor Tyro3, AXL, and proto‑oncogene tyrosine‑protein kinase Mer family of receptor tyrosine kinases, possessing multiple different functions in normal cells. Different therapeutic agents targeting AXL have been developed, typically including small molecule inhibitors, monoclonal antibodies, nucleotide aptamers, soluble receptors, and several natural compounds. AXL has emerged as a novel biomarker due to its role in biological processes and tumorigenesis.
The receptor tyrosine kinase AXL is emerging as a key player in tumor progression and metastasis and its expression correlates with poor survival in a plethora of cancers. The potential benefits of AXL inhibition for the treatment of metastatic cancers and additional roles for AXL in cancer progression are still being explored. Various approaches to inhibit AXL have been studied for cancer treatments including small molecule inhibitors that compete with ATP-binding or monoclonal antibodies. Indeed, several clinical trials are ongoing with the AXL inhibitor.
Further details related to country-based variations are provided in the report
AXL Receptor Tyrosine Kinase Inhibitors Epidemiology
The AXL receptor TKI epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total incident cases of selected indication for AXL receptor tyrosine kinase inhibitors, total eligible patient pool for AXL receptor tyrosine kinase inhibitors in selected indication, total treated cases in selected indication for AXL receptor tyrosine kinase inhibitors in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
- Among lung cancer cases, NSCLC accounts for approximately 85% of all diagnoses. This distribution highlights that NSCLC is the more prevalent form of lung cancer.
- In 2024, the total incident cases of NSCLC in 7MM was ~537,680, out of which the contribution of the US was ~38%.
- The total incident cases of HNSCC in the 7MM were approximately 150,900 in 2024, which is expected to increase in the upcoming years.
- According to the American Cancer Society (2024), an estimated 152,810 new cases of CRC were reported in 2024, with 81,540 cases in men and 71,270 cases in women in the US.
- In 2024, the American Cancer Society estimates approximately 310,720 new cases of invasive breast cancer among women in the US. TNBC represents 15% to 20% of all primary breast cancers and is the most aggressive subtype of breast cancer.
- In 2024, the total number of pNET incidence cases in the US was approximately 3,350.
- In 2024, the total Incident cases of AML were approximately 21,300 cases in the US, which is expected to grow during the forecast period.
- According to the National Cancer Institute, an estimated 80,980 new cases of RCC (kidney and renal pelvis) are expected in the US in 2025.
|
Target Pool Assessment of AXL Receptor Tyrosine Kinase Inhibitors | |
|
Indication |
Estimated Cases in the US (2024) |
|
NSCLC |
~204,850 (Incidence) |
|
HNSCC |
~63,750 (Incidence) |
|
pNET |
~3,350 (Incidence) |
|
AML |
~21,300 (Incidence) |
AXL Receptor Tyrosine Kinase Inhibitors Drug Chapters
The drug chapter segment of the AXL receptor TKI drugs market reports encloses a detailed analysis of AXL receptor TKI marketed drugs and pipeline drugs in different stages of development. It also helps understand the AXL receptor TKI’ clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed AXL Receptor Tyrosine Kinase Inhibitor Drugs
CABOMETYX (cabozantinib): Exelixis, Takeda Pharmaceuticals, Ipsen Pharma, and Bristol-Myers Squibb
Cabozantinib is a tyrosine kinase inhibitor used to treat advanced RCC, hepatocellular carcinoma (HCC), and medullary thyroid cancer. Cabozantinib suppresses metastasis, angiogenesis, and oncogenesis by inhibiting receptor tyrosine kinases. Cabozantinib inhibits the tyrosine kinase activity of MET, VEGFR-1, VEGFR-2 and VEGFR-3, AXL, RET, ROS1, TYRO3, MER, KIT, TRKB, FLT-3, and TIE-2.
- In March 2025, the US FDA approved Exelixis (cabozantinib) for adult and pediatric patients aged 12 years and older with previously treated, unresectable, locally advanced or metastatic, well-differentiated pNET and extra-pancreatic neuroendocrine tumors (epNET).
- The company secured clarity on the cabozantinib patent estate, supporting the continued commercial success of its CABOMETYX franchise in the US through 2030.
- Takeda Pharmaceuticals holds exclusive rights to develop and commercialize CABOMETYX in Japan, while Ipsen Pharma manages these rights outside the US and Japan; the drug is also being studied in collaboration with Bristol-Myers Squibb for combination trials with OPDIVO.
- CABOMETYX is recognized by the NCCN as a Category 1 subsequent-line treatment for HCC, with additional approvals from the EMA, Health Canada, and Japan’s PMDA further establishing its global role in managing advanced hepatocellular carcinoma.
Emerging AXL Receptor Tyrosine Kinase Inhibitors Drugs
Zanzalintinib: Exelixis and Merck
Zanzalintinib is a third-generation oral tyrosine kinase inhibitor that inhibits the activity of receptor tyrosine kinases implicated in cancer growth and spread, including VEGF receptors, MET, AXL and MER. These receptor tyrosine kinases are involved in both normal cellular function and in pathologic processes such as oncogenesis, metastasis, tumor angiogenesis and resistance to multiple therapies, including immune checkpoint inhibitors. The drug is currently being developed for the treatment of advanced solid tumors, including NET, genitourinary, CRC, and HNC.
In October 2024, Exelixis announced that the companies had entered into a clinical development collaboration to evaluate the combination of zanzalintinib with Merck’s anti-PD-1 therapy KEYTRUDA (pembrolizumab) in a Phase III pivotal trial for the treatment of patients with HNSCC, and zanzalintinib with WELIREG (belzutifan), in a Phase I/II trial and two Phase III pivotal trials for the treatment of patients with RCC.
Bemcentinib: BerGenBio
Bemcentinib is a first-in-class, selective, oral once-a-day inhibitor of AXL receptor tyrosine kinase (AXL) a promising therapeutic target for serious diseases. In cancer, recent studies have revealed a central role of AXL signaling in tumor proliferation, survival, metastasis, and resistance to therapy.
BerGenBio has completed a Phase II study (BGBC003) testing bemcentinib as a monotherapy and in combination with chemotherapy in AML. In collaboration with Merck, BerGenBio has conducted a Phase II study in recurrent NSCLC to study bemcentinib in combination with KEYTRUDA, the ICI therapy in NSCLC.
- In March 2025, BerGenBio announced the publication of a peer-reviewed article entitled “Bemcentinib as Monotherapy and in Combination with Low-Dose Cytarabine in Acute Myeloid Leukemia Patients Unfit for Intensive Chemotherapy” in the journal Nature Communications.
- In January 2025, BerGenBio announced the enrollment of the first patient in its clinical trial evaluating bemcentinib for advanced adenocarcinoma of the lung.
|
Comparison of Key Emerging AXL receptor Tyrosine Kinase Inhibitors (TKI) | ||||
|
Product |
Company |
RoA |
Phase |
Indication |
|
Zanzalintinib |
Exelixis and Merck |
Oral |
III; II/III; Ib/II |
CRC, non-clear RCC; HNSCC; multiple solid tumors |
|
Bemcentinib |
BerGenBio |
Oral |
II |
NSCLC, advanced adenocarcinoma lung cancer, AML, severe respiratory infections |
|
SLC-391 |
Signalchem Lifesciences and Merck |
Oral |
Ib/II |
NSCLC |
Note: Detailed current and emerging therapies assessment will be provided in the full report of AXL Receptor Tyrosine Kinase Inhibitors
AXL Receptor Tyrosine Kinase Inhibitors Drug Class Insights
AXL receptor TKI are a class of targeted therapies aimed at blocking the AXL signaling pathway, which is involved in key cancer processes such as cell survival, growth, metastasis, immune escape, and resistance to treatment. AXL, a member of the TAM receptor family (Tyro3, AXL, Mer), is often overexpressed in several malignancies, including NSCLC, RCC, and others. Notable drugs in this class include bemcentinib, SLC-391, and others which are currently under clinical investigation.
AXL Receptor Tyrosine Kinase Inhibitors Market Outlook
The receptor tyrosine kinase AXL is emerging as a key player in tumor progression and metastasis and its expression correlates with poor survival in a plethora of cancers. While studies have shown the benefits of AXL inhibition for the treatment of metastatic cancers, additional roles for AXL in cancer progression are still being explored. AXL Receptor Tyrosine Kinase Inhibitors, either as single agents or in combination with conventional chemotherapy or other inhibitors such as immune checkpoint inhibitors, angiogenesis inhibitors, and other TKIs, are likely to improve the survival of many patients.
Key AXL Receptor Tyrosine Kinase Inhibitors manufacturers, including Astellas Pharma, Exelixis, Signalchem Lifesciences, BerGenBio, and others, are involved in developing drugs for AXL Receptor Tyrosine Kinase Inhibitors for various indications such as metastatic cancer, advanced solid tumors, NSCLC, CRC, AML, and other malignancies.
AXL Receptor Tyrosine Kinase Inhibitors Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging AXL receptor TKI expected to be launched in the AXL Receptor Tyrosine Kinase Inhibitors market during 2025–2034.
AXL Receptor Tyrosine Kinase Inhibitors Pipeline Development Activities
The AXL Receptor Tyrosine Kinase Inhibitors market report provides insights into different therapeutic candidates in different phases of development. It also analyzes key AXL Receptor Tyrosine Kinase Inhibitors manufacturers involved in developing targeted therapeutics.
The presence of numerous drugs under different stages is expected to generate immense opportunity for AXL receptor TKI market growth over the forecasted period.
AXL Receptor Tyrosine Kinase Inhibitors Clinical Trials activities
The AXL Receptor Tyrosine Kinase Inhibitors clinical trials market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for AXL receptor TKI emerging therapies.
KOL Views on AXL Receptor Tyrosine Kinase Inhibitors
To keep up with current and future AXL Receptor Tyrosine Kinase Inhibitors market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on AXL receptor TKI' evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as the Centre for Cancer Biomarkers, American Lung Association, Carle Health, etc. were contacted.
Their opinion helps understand and validate current and emerging therapy treatment patterns or AXL Receptor Tyrosine Kinase Inhibitors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the AXL Receptor Tyrosine Kinase Inhibitors market and the unmet needs.
|
KOL Views |
|
“AXL is a critical mediator of epithelial–mesenchymal transition (EMT) and therapeutic resistance across many cancers. Inhibiting AXL can resensitize tumors to chemotherapy and immune checkpoint blockade.” MD, PhD, Anderson Cancer Center, US |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
AXL Receptor Tyrosine Kinase Inhibitors Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The AXL Receptor Tyrosine Kinase Inhibitors market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Key Updates on AXL Receptor Tyrosine Kinase Inhibitors Clinical Trials
- In May 2025, Exelixis announced the results from an expansion cohort of the Phase Ib/II STELLAR-002 trial evaluating zanzalintinib in combination with either OPDIVO or a fixed-dose combination of nivolumab and OPDUALAG (relatlimab) in patients with previously untreated advanced clear cell RCC at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting.
- In January 2025, Exelixis announced the results from an expansion cohort of the Phase Ib/II STELLAR-001 trial evaluating zanzalintinib alone or in combination with TECENTRIQ (atezolizumab) in patients with previously-treated mCRC at the ASCO 2025 Gastrointestinal Cancers Symposium.
- In February 2025, BerGenBio announced its decision to close its BGBC016 study of bemcentinib in combination with standard of care therapy in first line (1L) NSCLC patients with a mutation in the STK11 gene (STK11m).
- In February 2025, Exelixis announced the final results from the Phase III CheckMate -9ER pivotal trial evaluating CABOMETYX in combination with OPDIVO versus sunitinib for patients with previously untreated advanced RCC at at the American Society of Clinical Oncology 2025 Genitourinary Cancers Symposium (ASCO GU).
Scope of the AXL Receptor Tyrosine Kinase Inhibitors Market Report
- The AXL Receptor Tyrosine Kinase Inhibitors market report covers a segment of key events, an executive summary, and a descriptive overview of AXL receptor tyrosine kinase inhibitors, explaining its mechanism, and therapies (current and emerging).
- Comprehensive insight into the competitive landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the AXL receptor tyrosine kinase inhibitors market, historical and forecasted market size, and market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The AXL Receptor Tyrosine Kinase Inhibitors drugs market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis expert insights/KOL views, and treatment preferences that help shape and drive the 7MM AXL receptor tyrosine kinase inhibitors market.
AXL Receptor Tyrosine Kinase Inhibitors Market Report Insights
- AXL Receptor Tyrosine Kinase Inhibitors Targeted Patient Pool
- Therapeutic Approaches
- AXL Receptor Tyrosine Kinase Inhibitors Pipeline Analysis
- AXL Receptor Tyrosine Kinase Inhibitors Market Size and Trends
- Existing and future Market Opportunity
AXL Receptor Tyrosine Kinase Inhibitors Market Report Key Strengths
- Ten years Forecast
- The 7MM Coverage
- Key Cross Competition
- AXL Receptor Tyrosine Kinase Inhibitors Drugs Uptake
- Key AXL Receptor Tyrosine Kinase Inhibitors Market Forecast Assumptions
AXL Receptor Tyrosine Kinase Inhibitors Market Report Assessment
- Current Treatment Practices
- AXL Receptor Tyrosine Kinase Inhibitors Unmet Needs
- AXL Receptor Tyrosine Kinase Inhibitors Pipeline Product Profiles
- AXL Receptor Tyrosine Kinase Inhibitors Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint)
Key Questions
- What was the AXL receptor tyrosine kinase inhibitors total market size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for AXL Receptor Tyrosine Kinase Inhibitors market growth?
- Which AXL Receptor Tyrosine Kinase Inhibitors drug is going to be the largest contributor in 2034?
- What is the most lucrative market for AXL receptor tyrosine kinase inhibitors?
- Which AXL Receptor Tyrosine Kinase Inhibitors drug accounts for maximum AXL inhibitor sales?
- What are the pricing variations among different geographies for approved therapies?
- How the reimbursement landscape has for AXL receptor tyrosine kinase inhibitors evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with AXL receptor tyrosine kinase inhibitors? What will be the growth opportunities across the 7MM for the patient population of AXL Receptor Tyrosine Kinase Inhibitors?
- What are the key factors hampering the growth of the AXL receptor tyrosine kinase inhibitors market?
- What key designations have been granted to the therapies for AXL receptor tyrosine kinase inhibitors?
- What is the cost burden of approved AXL Receptor Tyrosine Kinase Inhibitor therapies on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved AXL Receptor Tyrosine Kinase Inhibitor therapies?
Reasons to buy
- The AXL Receptor Tyrosine Kinase Inhibitors therapeutics market report will help develop business strategies by understanding the latest trends and changing dynamics driving the AXL receptor tyrosine kinase inhibitors market.
- Understand the existing AXL Receptor Tyrosine Kinase Inhibitors market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming AXL Receptor Tyrosine Kinase Inhibitors companies in the AXL Receptor TKI market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing AXL Receptor Tyrosine Kinase Inhibitors market so that the upcoming AXL Receptor Tyrosine Kinase Inhibitors Companies can strengthen their development and launch strategy.