Cancer Vaccines Market
- The Cancer Vaccines Market is characterized by a healthy and growing pipeline including IO102-IO103 (IO Biotech), CAN-2409 (Candel Therapeutics), mRNA-4157 (V940) + KEYTRUDA (Merck and Moderna), PDC*lung01 (PDC*line Pharma/LG Chem), LB-LR1109 (LG Chem and AVEO Oncology) and others which are designed to target a wide range of cancers including Non-Small Cell Lung Cancer (NSCLC), colorectal cancer, glioblastoma, melanoma, breast cancer, and others.
- The steady advancement in vaccine development over the past few decades, leading to the creation of vaccines targeting cancers, is poised to transform the way different types of cancer are treated.
- Bacillus Calmette-Guérin (BCG) became the first immunotherapy of any type to be approved by the FDA and is still used for the treatment of early-stage bladder cancer. In 2010, PROVENGE (sipuleucel-T) was approved by the US FDA for the treatment of advanced prostate cancer followed by the approval of IMLYGIC (talimogene laherparepvec) in 2015 for treatment of melanoma.
- Recent developments in RNA technology and the recognition of the clinical importance of tumor neoantigens have led to encouraging trials of novel RNA vaccines and provide hope that effective cancer vaccines will be widely available in the next few years
- Several Cancer Vaccines Companies such as WestGene Biopharma, IO Biotech, PDS Biotechnology, Merck and Moderna, Dendreon Pharmaceuticals, and BioVex are engaged in the development of cancer vaccines, with a range of approved and emerging drugs.
- At the American Association for Cancer Research (AACR 2024), PDC*line Pharma, BioNTech, and others presented the data on various cancer vaccines that are being developed for different cancers.
- In July 2024, IO Biotech revealed that findings from a Phase II basket trial evaluating IO102-IO103 in combination with pembrolizumab will be showcased at the ESMO Congress 2024. The presentation will include data from the complete squamous cell carcinoma of the head and neck (SCCHN) cohort of the IOB-022/KN-D38 study.
- In June 2024, LG Chem and AVEO Oncology announced the enrollment of the first patient in the U.S. for a Phase I clinical trial of LB-LR1109 (NCT06332755; LG project code LR19155), LG Chem’s first proprietary anti-cancer investigational drug. The study will evaluate the drug in participants with unresectable non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), renal cell carcinoma (RCC), urothelial carcinoma, and malignant melanoma.
- In May 2024, Candel Therapeutics presented Phase II trial data at the 2024 ASCO Annual Meeting, highlighting prolonged overall survival with CAN-2409 in non-small cell lung cancer (NSCLC) patients.
- In April 2024, CAN-2409 was granted Orphan Drug Designation by the US FDA for the treatment of pancreatic cancer.
- Cancer vaccines, although have not yet demonstrated a significant clinical impact, possess a huge potential for therapeutic purposes. Being tested as monotherapy as well as in combination with IOs, the class can confer long-lasting immunity against cancer recurrence and hold promise as a crucial component of future cancer treatment armamentarium.
- The future direction of vaccine development will likely rely on combinatorial approaches with complementary anticancer therapeutics. For example, combinations with ICIs have already shown notable potential.
Request for Unlocking the Sample Page of the Cancer Vaccines Market
DelveInsight’s “Cancer Vaccines Market Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of cancer vaccines, historical and Competitive Landscape as well as the cancer vaccines therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
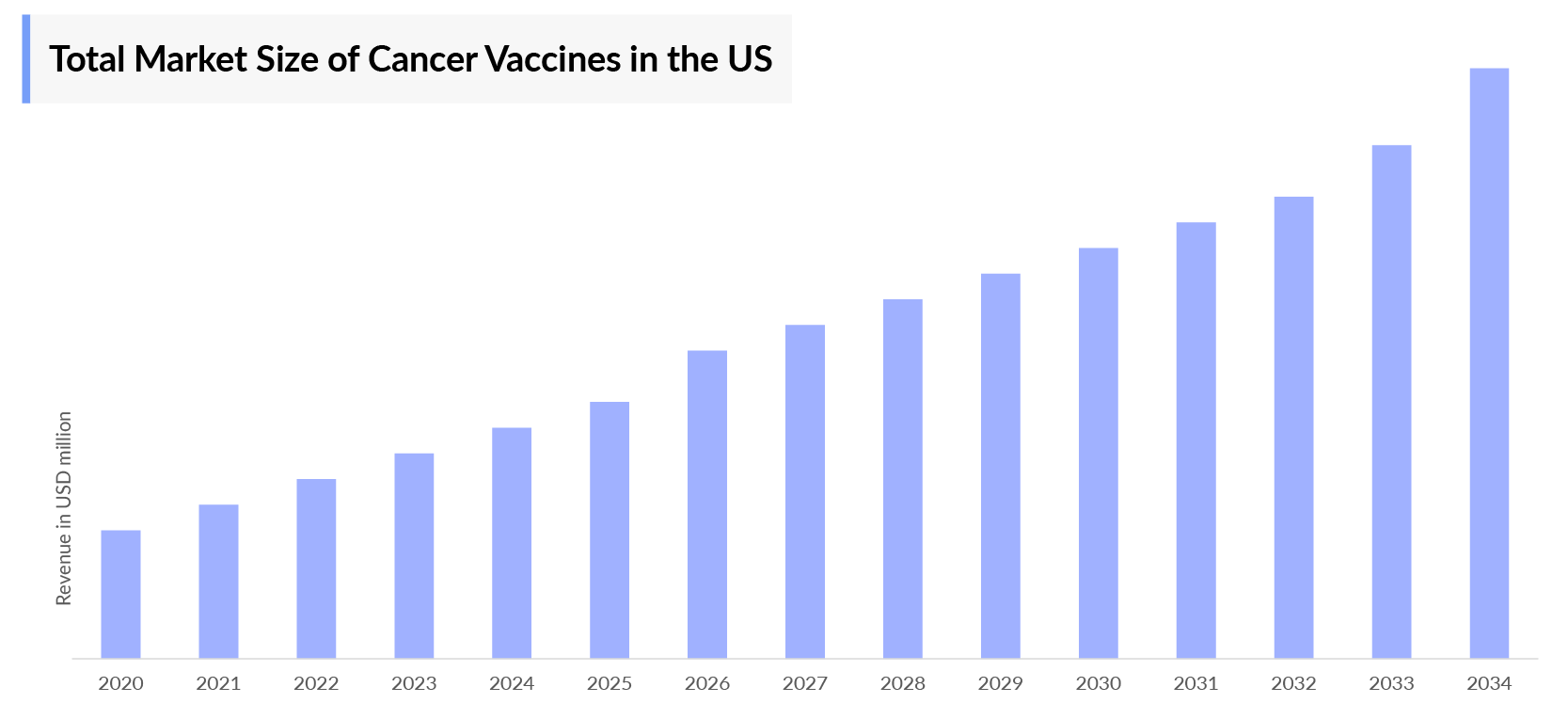
The Cancer Vaccines Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM cancer vaccines’ market size from 2020 to 2034. The report also covers current cancer vaccine treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Cancer Vaccines Market |
|
|
Cancer Vacciness Market Size | |
|
Cancer Vaccines Companies |
IO Biotech, Merck, Moderna, Candle Therapeutics, PDS Biotechnology, PDC*line Pharma, LG Chem, AVEO Oncology, ISA Pharmaceuticals, Archival Farma, and others. |
|
Cancer Vaccines Epidemiology Segmentation |
|
Cancer Disease Treatment Market
Cancer vaccines, a form of immunotherapy, assist the immune system in recognizing and eliminating cancer cells. They may originate from a patient's tumor cells, dendritic cells, or particular proteins in cancer cells. These vaccines can be divided into two types - preventive and therapeutic. Preventive vaccines are more established and aim at preventing viral infections linked to potential future cancers. Therapeutic vaccines, in contrast, target existing cancer, addressing a diverse array of cancer types, locations, and even personalized antigens unique to an individual's cancer.
Cancer treatment vaccines treat cancer by strengthening the body’s natural defenses against the cancer. Unlike cancer prevention vaccines, cancer treatment vaccines are designed to be used in people who already have cancer—they work against cancer cells, not against something that causes cancer. The idea behind treatment vaccines is that cancer cells contain substances, called tumor-associated antigens that are not present in normal cells or, if present, are at lower levels. Treatment vaccines can help the immune system learn to recognize and react to these antigens and destroy cancer cells that contain them.
Further details related to country-based variations are provided in the report…
 Cancer Vaccines Epidemiology
Cancer Vaccines Epidemiology
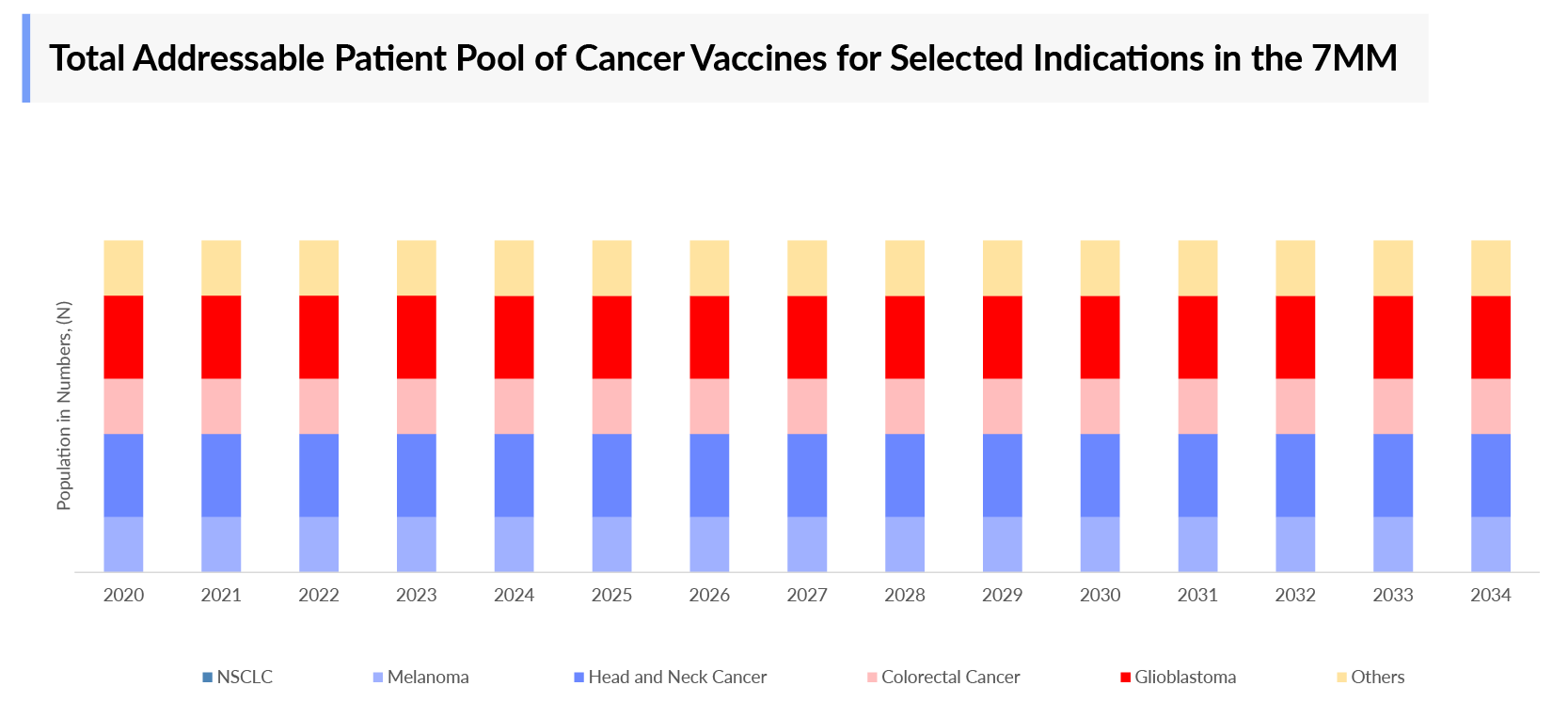
The cancer vaccines’ epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total incident cases of selected indications for cancer vaccines, total eligible patient pool of selected indications for cancer vaccines, total treated cases in selected indications for cancer vaccines in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
- The estimated NSCLC Incidence Cases in the US in 2023 were nearly 200,000.
- Uveal melanoma affects between 500 and 600 patients in the UK every year.
- In 2023, Germany had nearly 4,000 cases of glioblastoma.
- Children in the age group 0-14 have been observed to have an incident rate of nearly 15 for neuroblastoma in Japan.
Cancer Vaccines Drug Chapters
The drug chapter segment of the cancer vaccines therapeutics market reports encloses a detailed analysis of marketed cancer vaccines and late-stage (Phase III and Phase II) Cancer Vaccines pipeline drugs analysis. It also helps understand the Cancer Vaccines clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Cancer Vaccines news and press releases. The clinical trials of drugs and vaccines market is expanding rapidly, driven by innovations, regulatory advancements, and increasing global healthcare demands.
Cancer Vaccines Marketed Drugs
- PROVENGE (sipuleucel-T): Dendreon Pharmaceuticals
PROVENGE (sipuleucel-T), developed by Dendreon Pharmaceuticals, is an autologous cellular immunotherapy available as a suspension for intravenous infusion. PROVENGE consists of autologous peripheral blood mononuclear cells, including antigen-presenting cells (APCs) that have been activated during a defined culture period with a recombinant human protein, PAP-GM-CSF, consisting of prostatic acid phosphatase (PAP), an antigen expressed in prostate cancer tissue, linked to granulocyte-macrophage colony-stimulating factor (GM-CSF), an immune cell activator.
It was approved by the US FDA in 2010 for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant (hormone-refractory) prostate cancer but it was hindered by its hefty price tag and uncertainty over insurance coverage and the company went bankrupt in 2014.
|
Table 1: Comparison of Key Approved Vaccines for Cancer Prevention and Treatment | |||
|
Product |
Company |
RoA |
Indication |
|
GARDASIL 9 |
Merck Sharp & Dohme |
IM |
· Cervical, vulvar, vaginal, and anal cancer caused by HPV types 16 and 18 · Genital warts (condyloma acuminate) caused by HPV types 6 and 11 |
|
IMLYGIC |
BioVex |
Intralesional |
· local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery |
|
HEPLISAV-B |
Dynavax Technologies |
IM |
· Hepatitis B virus |
|
PROVENGE |
Dendreon Pharmaceuticals |
IV |
· symptomatic metastatic castrate-resistant (hormone-refractory) prostate cancer |
Note: Detailed current therapies assessment will be provided in the full report of cancer vaccines.
Cancer Vaccines Emerging Drugs
- IO102-IO103: IO Biotech
IO102-IO103 is an investigational therapeutic cancer vaccine designed to kill both tumor cells and immune-suppressive cells in the tumor microenvironment (TME) by stimulating activation and expansion of T cells against indoleamine 2,3-dioxygenase (IDO) and/or programmed death-ligand 1 (PD-L1) cells.
In July 2024, IO Biotech announced that an abstract related to IO102-IO103, has been accepted for poster presentation at the European Society for Medical Oncology (ESMO) Congress 2024. The poster will share data from one of the Phase II basket trials of IO102-IO103 in combination with pembrolizumab. The data for the complete squamous cell carcinoma of the head and neck (SCCHN) cohort of the IOB-022/KN-D38 study will be presented at the Congress.
- CAN-2409: Candel Therapeutics
CAN-2409 is an investigational off-the-shelf replication-defective adenovirus designed to deliver the herpes simplex virus thymidine kinase (HSV-tk) gene to a patient’s tumor and induce a systemic anti-tumor immune response. Currently, Candel is evaluating the effects of treatment with CAN-2409 in NSCLC, borderline resectable PDAC, and localized, non-metastatic prostate cancer.
In April 2024, CAN-2409 was granted Orphan Drug Designation by the US FDA for the Pancreatic Cancer treatment. It has also been previously granted Fast Track Designation by the FDA for the treatment of PDAC, stage III/IV NSCLC. In May 2024, Candel Therapeutics reported prolonged overall survival in the Phase II Trial of CAN-2409 for NSCLC at the 2024 ASCO Annual Meeting. Previously, In April 2024, the company also announced positive interim data from the Phase II clinical trial of CAN-2409 in non metastatic pancreatic cancer. Candel’s pivotal prostate cancer clinical trials Phase III is being conducted under a Special Protocol Assessment by FDA for which data is anticipated in Q4 2024.
|
Table 2: Comparison of key emerging drugs | ||||
|
Drug name |
Company |
MoA |
Phase |
Indication |
|
IO102-IO103 |
IO Biotech |
Immune checkpoint inhibition |
III |
Advanced melanoma and solid tumors |
|
mRNA-4157 (V940) + KEYTRUDA |
Merck and Moderna |
Immune checkpoint inhibition |
III |
Melanoma, NSCLC, cSCC, bladder cancer and RCC |
|
CAN-2409 |
Candle Therapeutics |
Oncolytic virus gene therapy |
II |
PDAC, stage III/IV NSCLC, non-metastatic pancreatic cancer and prostate cancer |
|
Nelipepimut-S + Cemiplimab |
ISA Pharmaceuticals |
PD-1/PD-L1 inhibitor |
II |
Oropharyngeal cancer |
|
PDS0101 + Pembrolizumab |
PDS Biotechnology |
PD-1 inhibitor |
II |
HPV16-positive head and neck cancer |
|
PDC*lung01 |
PDC*line Pharma/LG Chem |
CD47 antigen inhibitors |
I/II |
NSCLC |
|
LB-LR1109 |
LG Chem and AVEO Oncology |
LILRB1 inhibitor |
I |
Solid tumors |
|
RUTI |
Archival Farma |
TNF-α and IL-1β suppressor |
I |
non-muscle-invasive bladder cancer |
Cancer Vaccine Market Outlook
The Cancer Vaccines Market is expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed with different types of cancers, the growing awareness of cancer vaccines, and the increasing number of cancer vaccines that are under clinical trials and filed for approval by various companies.
Bacillus Calmette-Guérin (BCG) became the first immunotherapy of any type to be approved by the FDA and is still used for the treatment of early-stage bladder cancer. In 2010, PROVENGE (sipuleucel-T) was approved by the US FDA for the treatment of advanced prostate cancer followed by the approval of IMLYGIC (talimogene laherparepvec) in 2015 for the treatment of melanoma. The current cancer vaccines, under investigation, like IO102-IO103 (IO Biotech), mRNA-4157 (V940) + KEYTRUDA (Merck and Moderna), and others offer potential effects of therapy and prevention, addressing solid tumors, NSCLC, RCC, cSCC etc. With ongoing clinical trials and regulatory advancements, the Cancer Vaccines Market Outlook is promising, fostering continued innovation and progress in NSCLC. This evolution holds the potential to improve patient outcomes and redefine standards of care in NSCLC management globally. Overall, this is exciting with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of cancer vaccines and define their role in the therapy of cancer.
European Society for Medical Oncology (ESMO) Oral Presentations 2024
|
Drug name |
Company |
Title |
|
IO102-IO103 |
IO Biotech |
A phase II trial of the IO102-IO103 vaccine plus pembrolizumab: Completed cohort for first-line (1L) treatment of advanced squamous cell carcinoma of the head and neck (SCCHN) |
|
RUTI |
Archival Farma |
RUTIVAC-1 study: A randomized, double-blind, placebo-controlled phase I trial to evaluate the immunomodulatory effect of RUTI in individuals with high-risk non-muscle-invasive bladder cancer treated with intravesical BCG |
|
PDS0101 + Pembrolizumab |
PDS Biotechnology |
VERSATILE-002: Survival with First-Line Treatment with PDS0101 Therapeutic Vaccine and Pembrolizumab in HPV16-positive Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (HNSCC) |
|
EBV mRNA vaccine (WGc-043 injection) |
WestGene Biopharma |
An exploratory study to assess the safety, immunogenicity, and preliminary anti-tumor activity of the EBV mRNA vaccine (WGc-043 injection) in patients with NK/T cell lymphoma |
*Full details about abstracts will be provided in the full report.
Cancer Vaccines Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging cancer vaccines market is expected to be launched in the market during 2024–2034. The new generation cancer vaccine market is rapidly evolving, driven by advanced immunotherapies, innovative research, clinical trials, and growing global demand.
Cancer Vaccines Pipeline Development Activities
The Cancer Vaccines therapeutics market report provides insights into different Cancer Vaccines clincial trials within Phase III, Phase II, and Phase I. It also analyzes key Cancer Vaccines Companies involved in developing targeted therapeutics. The presence of numerous drugs under different stages is expected to generate immense opportunity for cancer vaccines’ market growth over the forecasted period.
Pipeline Development Activities
The Cancer Vaccines therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for cancer vaccine therapies.
KOL Views
To keep up with current and future market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on cancer vaccines' evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as Baptist Health Medical Group and others. Their opinion helps understand and validate current and emerging therapy treatment patterns or cancer vaccine market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
KOL Views |
|
“Selection of the most efficacious context in which to use a cancer vaccine is important as well. Although cancer vaccines have largely been studied in the adjuvant and metastatic settings, the neoadjuvant space remains relatively underexplored. Used as a neoadjuvant treatment, patients would typically not be faced with biologic barriers such as T-cell exhaustion, diminished immune system, and immunoediting from previous lines of therapy.” |
Cancer Vaccines Therapeutics Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Cancer Vaccines Therapeutics Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Key Updates on Cancer Vaccines
- In July 2024, IO Biotech announced that data from one of the Phase II basket trials of IO102-IO103 in combination with pembrolizumab will be presented at the ESMO Congress 2024. The data for the complete squamous cell carcinoma of the head and neck (SCCHN) cohort of the IOB-022/KN-D38 study.
- In June 2024, LG Chem and AVEO Oncology announced that it has enrolled the first patient in the US for a Phase I clinical study of LB-LR1109 (NCT06332755; LG project code LR19155), LG Chem’s first proprietary anti-cancer investigational drug candidate, in participants with unresectable and NSCLC, head and neck squamous cell carcinoma, RCC, urothelial carcinoma, or malignant melanoma.
- In June 2024, LG Chem and AVEO Oncology announced that it has enrolled the first patient in the US for a Phase I clinical study of LB-LR1109 (NCT06332755; LG project code LR19155), LG Chem’s first proprietary anti-cancer investigational drug candidate, in participants with unresectable and NSCLC, head and neck squamous cell carcinoma, RCC, urothelial carcinoma, or malignant melanoma.
The abstract list is not exhaustive, will be provided in the final report...
Cancer Vaccines Therapeutics Market Report Scope
- The Cancer Vaccines therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining their mechanism, and indications (current and emerging).
- Comprehensive insight into the competitive landscape, the future growth potential of cancer vaccines have been provided.
- Additionally, an all-inclusive account of the current and emerging vaccines and the elaborative profiles of late-stage and prominent vaccines that will impact the current Cancer Vaccines treatment market landscape.
- A detailed review of the cancer vaccines therapeutics market, historical and forecasted Cancer Vaccines treatment market size, Cancer Vaccines drugs market share by therapies and indications, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Cancer Vaccines therapeutics market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM cancer vaccines drugs market.
Cancer Vaccines Therapeutics Market Report Insights
- Cancer Vaccines Targeted Patient Pool
- Cancer Vaccines Therapeutic Approaches
- Cancer Vaccines Pipeline Drugs Analysis
- Cancer Vaccines Market Size and Trends
- Existing and future Cancer Vaccines Drugs Market Opportunity
Cancer Vaccines Therapeutics Market Report Key Strengths
- 11 Years Cancer Vaccines Market Forecast
- The 7MM Coverage
- Key Cross Competition
- Cancer Vaccines Drugs Uptake
- Key Cancer Vaccines Market Forecast Assumptions
Cancer Vaccines Therapeutics Market Report Assessment
- Current Cancer Vaccines Treatment Market Practices
- Cancer Vaccines Unmet Needs
- Cancer Vaccines Pipeline Drugs Analysis Profiles
- Cancer Vaccines Drugs Market Attractiveness
- Qualitative Analysis (SWOT)
- Cancer Vaccines Market Drivers
- Cancer Vaccines Market Barriers
Key Questions Answered In The Cancer Vaccines Market Report:
- What was the cancer vaccines therapeutics market size, the Cancer Vaccines treatment market size by therapies, Cancer Vaccines drugs market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative market and indication for cancer vaccines?
- What are the pricing variations among different geographies for approved therapies?
- How the reimbursement landscape for cancer vaccines evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with cancer vaccines? What will be the growth opportunities across the 7MM for the patient population of cancer vaccines?
- What are the key factors which can hamper the growth of the cancer vaccines market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for cancer vaccines?
- What is the cost burden of approved vaccines on the patient?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
Reasons to Buy Cancer Vaccines Market Forecast Report
- The Cancer Vaccines therapeutics market report will help develop business strategies by understanding the latest trends and changing dynamics driving the cancer vaccines drugs market.
- Understand the existing Cancer Vaccines therapeutics market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Cancer Vaccines therapeutics market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Cancer Vaccines therapeutics market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles @ Latest DelveInsight Blogs