CRISPR Market Forecast
- The CRISPR therapies market is expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness of CRISPR therapies, the increasing number of CRISPR therapies that are under clinical trials, and the increasing interest of major pharmaceutical companies toward it.
- In December 2023, the US FDA gave its approval for two groundbreaking gene editing therapies. The first one, CASGEVY (exagamglogene autotemcel), is a CRISPR-based therapy developed jointly by Vertex Pharmaceuticals and CRISPR Therapeutics. The second therapy, LYFGENIA (lovotibeglogene autotemcel), was created by Bluebird bio. Both of these therapies represent significant advancements in the field of gene editing.
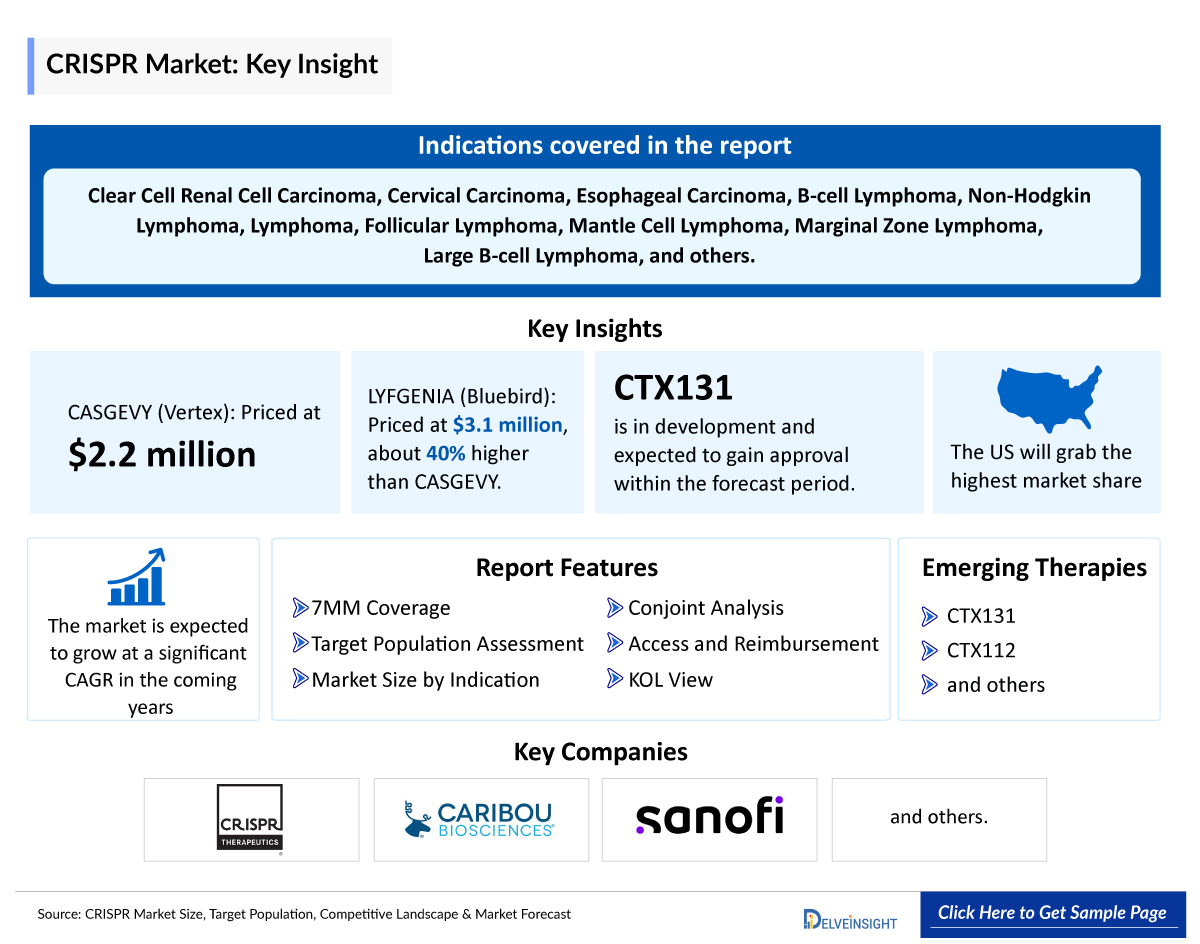
- Vertex Pharmaceuticals has priced CASGEVY at USD 2.2 million and Bluebird is pricing LYFGENIA at a wholesale acquisition cost of USD 3.1 million, about 40% higher than that of CASGEVY.
- CRISPR therapies is a form of genetic editing which offers tremendous potential for addressing various diseases by precisely targeting specific genes associated with those diseases.
- Currently, CRISPR therapies is approved for the treatment of transfusion-dependent β-thalassemia and sickle cell disease (includeing in patients with a history of vaso-occlusive events).
- Several CRISPR therapies are currently being evaluated in clinical trials. An example is CRISPR Therapeutic’s, CTX131 which is in the developmental stage and is anticipated to receive approval during the forecast period.
- During the ASCO 2024 annual meeting, CRISPR therapeutics is expected to present the results of its clinical studies conducted on CTX131.
- In April 2023, FDA has granted a fast track designation to CB-011, a CRISPR-edited allogeneic CAR T-cell therapies developed by Caribou Biosciences, for the treatment of patients with relapsed/refractory multiple myeloma.
- In May 2023, Prevail Therapeutics, a subsidiary of Lily, secured exclusive rights to Scribe Therapeutics’ CRISPR X-Editing (XE) technologies for USD 1.57 billion. This licensing agreement, aimed at developing in vivo therapies directed to specified targets known to cause serious neurological and neuromuscular diseases, stands as the largest CRISPR-based deal of the year.
- In July 2023, Sanofi expanded its collaboration with Scribe with a deal worth up to USD 1.24 billion, focusing on leveraging Scribe’s XE genome editing technologies for the development of in vivo therapies, particularly sickle cell disease and other genomic disorders.
- Crispr therapeutics, Caribou biosciences, Sanofi and others, and several other CRISPR companies are currently engaged in the development and production of CRISPR, which has the potential to significantly impact and enhance the CRISPR therapies market.
DelveInsight’s “ CRISPR Therapies Marke Size, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the CRISPR therapies, historical and Competitive Landscape as well as the CRISPR therapies market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The CRISPR therapies market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM CRISPR therapies market size from 2020 to 2034. The report also covers current CRISPR therapies treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
CRISPR Therapies Market: Understanding and Treatment Algorithm
CRISPR Therapies Overview
CRISPR stands for “Clustered Regularly Interspaced Short Palindromic Repeats”. It is a technology that allows scientists to make precise changes to an organism's DNA.
CRISPR therapies is a form of genetic editing that holds incredible promise for treating a wide range of diseases.
In this therapy, a CRISPR system is used to target specific genes associated with certain diseases. By doing so, either genetic mutations that cause disease can be corrected or genes can be modified to enhance beneficial traits. The process of CRISPR therapies involves delivering the CRISPR system into cells, where it can then make the desired changes to the DNA.
This therapy has the potential to revolutionize medicine by offering treatments for genetic disorders like cystic fibrosis and sickle cell disease, as well as providing new approaches for cancer treatment and even agricultural improvements.
CRISPR-Cas systems have become the most widely used genome editing technology in molecular biology laboratories all around the world due to its advantages of simple design, low cost, high efficiency, good repeatability and short-cycle.
CRISPR Therapies Treatment
Currently regulatory bodies have approved CRISPR therapies for the treatment of various types of sickle cell disease and transfusion-dependent β-thalassemia (TDT).
After the infusion of CASGEVY, the edited CD34+ cells engraft in the bone marrow and differentiate to erythroid lineage cells with reduced BCL11A expression. Reduced BCL11A expression results in an increase in γ-globin expression and HbF protein production in erythroid cells. In patients with severe sickle cell disease, HbF expression reduces intracellular hemoglobin S (HbS) concentration, preventing the red blood cells from sickling and addressing the underlying cause of disease, thereby eliminating vaso-occlusive crisis. In patients with transfusion-dependent β-thalassemia, γ-globin production improves the α-globin to non-α-globin imbalance thereby reducing ineffective erythropoiesis and hemolysis and increasing total hemoglobin levels, addressing the underlying cause of disease, and eliminating the dependence on regular red blood cell (RBC) transfusions.
Other Few CRISPR therapies which are in clinical development are CTX131, CTX112 and others.
CRISPR Therapies Drug Chapters
The drug chapter segment of the CRISPR therapies market reports encloses a detailed analysis of CRISPR therapies marketed drugs and late-stage (Phase II and Phase I) pipeline drugs. It also helps understand the CRISPR therapies' clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug the latest news and press releases.
Marketed CRISPR Drugs
CASGEVY (exagamglogene autotemcel): Vertex pharamceuticals/CRISPR Therapeutics
Casgevy, developed by Vertex Pharmaceuticals of Boston and CRISPR Therapeutics of Switzerland, is the first medicine available in the United States to treat a genetic disease using the CRISPR gene-editing technique.
CASGEVY (exagamglogene autotemcel) is a cellular gene therapy consisting of autologous CD34+ HSCs edited by CRISPR/Cas9-technology at the erythroid specific enhancer region of the BCL11A gene.
Casgevy involves the use of CRISPR-Cas9 to inactivate BCL11A expression in the precursors of red blood cells, thus releasing the handbrake on fetal globin expression, which in turn compensates for the sickle cell disease mutation in the β-globin gene.
LYFGENIA (lovotibeglogene autotemcel): Bluebird bio
LYFGENIA is a one-time gene therapy to treat sickle cell disease in patients 12 years of age or older and a history of vaso-occlusive events. LYFGENIA is made specifically for each patient, using the patient’s own blood stem cells (from which red blood cells are produced). It adds functional copies of the beta-globin gene into patients’ hematopoietic stem cells (HSCs) through transduction of autologous CD34+ cells with BB305 LVV leading to production of anti-sickling hemoglobin that may decrease or stop vaso-occlusive events.
|
Product |
Company |
Indication |
|
CASGEVY (exagamglogene autotemcel) |
Vertex/CRISPR |
|
|
LYFGENIA (lovotibeglogene autotemcel) |
Bluebird Bio |
Treatment of patients 12 years of age or older with sickle cell disease and a history of vaso-occlusive events. |
Note: Detailed current therapies assessment will be provided in the full report of CRISPR therapies
In both above mentioned therapies, stem cells are removed from a patient's blood for treatment. With Casgevy, CRISPR gene-editing technology knocks out a gene that triggers the development of defective, crescent-shaped blood cells. Meanwhile, medicine kills off flawed blood-producing cells in patients, who are then given back their own altered stem cells. The same thing happens with Lyfgenia, only a virus is used to deliver a genetic payload that causes the blood cells to start producing healthy hemoglobin. The genetically modified blood stem cells are then given back to the patient as a one-time, single-dose infusion.
Emerging CRISPR Drugs
CTX131: CRISPR Therapeutics
CTX131 is the investigational allogeneic CRISPR/Cas9 gene-edited CAR T cell therapy (Anti-CD70 allogeneic CAR T). It is in the development for the treatment of solid tumors and hematological malignancies that incorporates novel edits designed to enhance CAR T potency and reduce CAR T exhaustion.
CTX112: CRISPR Therapeutics
CTX112 is the investigational allogeneic CRISPR/Cas9 gene-edited CAR T cell therapy (Anti-CD19 allogeneic CAR T) in development for the treatment of CD19+ malignancies and autoimmune diseases that incorporates novel edits designed to enhance CAR T potency and reduce CAR T exhaustion.
CTX131 is the investigational allogeneic CRISPR/Cas9 gene-edited CAR T cell therapy (Anti-CD70 ALLOGENIC cart). It is in the development for the treatment of solid tumors and hematological malignancies that incorporates novel edits designed to enhance CAR T potency and reduce CAR T exhaustion.
Note: Detailed emerging therapies assessment will be provided in the final report.
|
List of Emerging Drugs | |||||
|
CTX131 |
CRISPR therapeutics |
Relapsed or Refractory Solid Tumors (Clear Cell Renal Cell Carcinoma, Cervical Carcinoma, Esophageal Carcinoma)
|
CRISPR therapies |
I/II |
NCT05795595 |
|
CTX112 |
CRISPR therapeutics |
Relapsed or Refractory B-Cell Malignancies (B-cell Lymphoma, Non-Hodgkin Lymphoma, B-cell Malignancy, Chronic Lymphocytic Leukemia /Small Lymphocytic Lymphoma, Follicular Lymphoma, Mantle Cell Lymphoma, Marginal Zone Lymphoma, Large B-cell Lymphoma) |
CRISPR therapies |
I/II |
NCT05643742 |
Note: The emerging drug list is indicative, the full list will be given in the final report.
CRISPR Therapies Market Outlook
The CRISPR therapies market is expected to grow significantly in the coming years. This is due to the increasing number of patients who are being diagnosed with cancer, the growing awareness of CRISPR therapies, the increasing number of CRISPR therapies that are under clinical trials, and the increasing interest of major pharmaceutical companies toward it.
In December 2023, CASGEVY and LYFGENIA, the first CRISPR-based gene therapies have received approval from the Food and Drug Administration (FDA) for sickle cell anemia and beta-thalassemia treatment.
These two therapies work in different ways however both therapies utilise the Nobel-winning CRISPR/Cas 9 genome editing technology. The two therapies are both one-time infusions. LYFGENIA uses a lentiviral vector to introduce genetic modifications into the patient’s blood stem cells to produce a type of hemoglobin A to fill in for dysfunctional ones, By comparison, CASGEVY uses CRISPR tech to edit blood stem cells to increase the production of fetal hemoglobin.
Bluebird is pricing LYFGENIA at a wholesale acquisition cost of USD 3.1 million, about 40% higher than the USD 2.2 million list price Vertex has placed on its CRISPR Therapeutics-partnered CASGEVY.
Besides the higher price, LYFGENIA comes with a black box warning highlighting the risk of hematologic malignancy. Vertex’s CASGEVY approval didn't come with a boxed warning.
Before the FDA’s approvals, a physician survey conducted by J.P. Morgan showed that 80% of doctors saw no difference between LYFGENIA and CASGEVY.
Several key players, including CRISPR therapeutics, Caribou biosciences and others, are involved in developing drugs for CRISPR therapies for various indications such as Pancreatic Adenocarcinoma, Malignant Pleural Mesothelioma, B-cell Lymphoma, Non-Hodgkin Lymphoma and others.
Overall, this is an exciting new class of agents with great potential for development. The maturation of current studies over the next few years will lead to a better understanding of CRISPR therapies and define their role in the therapies of cancer.
CRISPR Therapies Drugs Uptake
This section focuses on the uptake rate of potential approved and emerging CRISPR therapies expected to be launched in the CRISPR therapies market during 2020–2034.
CRISPR Therapies Pipeline Development Activities
The CRISPR Therapies market report provides insights into different therapeutic candidates in Phase II, and Phase I. It also analyzes key CRISPR companies involved in developing targeted therapeutics.
The presence of numerous drugs under different stages is expected to generate immense opportunity for CRISPR therapies market growth over the forecasted period.
CRISPR Clinical Trial Activities
The CRISPR Market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for CRISPR therapies emerging therapies.
The increasing strategic collaborations among major market players to enhance the growth of their pipeline products are anticipated to drive market expansion. For example, Sanofi announced a strategic collaboration with Scribe Therapeutics for the use of Scribe’s CRISPR genome editing technologies to enable genetic modification of novel natural killer (NK) cell therapies for cancer.
Under the terms of the agreement, Scribe will receive USD 25 million in upfront payment and be eligible to potentially receive more than USD 1 billion in payments based on development and commercial milestones, as well as tiered royalties on net future sales on any products that may result from this research agreement.
Concurrently, in July 2023, Sanofi expanded its collaboration with Scribe with a deal worth up to USD 1.24 billion, focusing on leveraging Scribe’s XE genome editing technologies for the development of in vivo therapies, particularly sickle cell disease and other genomic disorders.
In December 2017, Crispr therapeutics announced that it will co-develop and co-commercialize CTX001 for the treatment of hemoglobinopathies, including β-thalassemia and sickle cell disease with Vertex Pharmaceuticals.
KOL Views on CRISPR
To keep up with current and future CRISPR market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on CRISPR therapies' evolving treatment landscape, patient reliance on conventional therapies, patient therapies switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 25+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as Dana-Farber Cancer Institute and others.
Their opinion helps understand and validate current and emerging therapies treatment patterns or CRISPR therapies inhibitors market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the CRISPR market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
CRISPR Market Access and Reimbursement
Reimbursement for CRISPR therapies has been universal in the United States and even in Europe.
Authorities in the U.K. have preliminarily recommended that CASGEVY (exagamglogene autotemcel) not be reimbursed for eligible patients with sickle cell disease (SCD) under its national public health program based on uncertainties about the gene therapies’s cost-effectiveness.
In United States, CASGEVY and LYFGENIA have been reimbursed by Anthem and others.
The CRISPR market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Key Updates on CRISPR Therapies
- Many Pharma companies will be presenting the data of their CRISPR therapies during the ASCO 2024 conference including CRISPR therapeutics and others. During the ASCO 2024 annual meeting, CRISPR therapeutics is expected to present the results of clinical trial NCT05795595, with the title “A phase 1/2, open-label, multicenter, dose escalation and cohort expansion study of the safety and efficacy of anti-CD70 allogeneic CRISPR-Cas9–engineered T cells (CTX131) in adult patients with relapsed or refractory solid tumors” (Abstract # TPS2676).
- The FDA has awarded fast track designation to CB-011, an allogeneic CAR T-cell therapy edited using CRISPR technology, developed by Caribou Biosciences. This designation is for treating patients with relapsed/refractory multiple myeloma.
- Prevail Therapeutics, a subsidiary of Lily, obtained exclusive rights to Scribe Therapeutics' CRISPR X-Editing (XE) technologies for a staggering sum of USD 1.57 billion. This licensing agreement is focused on creating in vivo therapies tailored to specific targets associated with severe neurological and neuromuscular disorders, marking the most substantial CRISPR-based deal of the year
The abstract list is not exhaustive, will be provided in the final report
Scope of the CRISPR Market Report
- The CRISPR market report covers a segment of key events, an executive summary, and a descriptive overview of CRISPR therapies, explaining its mechanism, and therapies (current and emerging).
- Comprehensive insight into the Competitive Landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the CRISPR therapies market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The CRISPR market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis expert insights/KOL views, and treatment preferences that help shape and drive the 7MM CRISPR therapies market.
CRISPR Therapies Market Report Insights
- CRISPR Therapies Targeted Patient Pool
- CRISPR Therapies Therapeutic Approaches
- CRISPR Therapies Pipeline Analysis
- CRISPR Therapies Market Size
- CRISPR Market Trends
- Existing and Future CRISPR Market Opportunity
CRISPR Therapies Market Report Key Strengths
- Eleven years Forecast
- The 7MM Coverage
- Key Cross Competition
- CRISPR Drugs Uptake
- Key CRISPR Market Forecast Assumption
CRISPR Therapies Market Report Assessment
- Current Treatment Practices
- CRISPR Unmet Needs
- CRISPR Pipeline Product Profiles
- CRISPR Market Attractiveness
- Qualitative Analysis (SWOT)
Key Questions
- What was the CRISPR therapies market size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which CRISPR is going to be the largest contributor in 2034?
- Which is the most lucrative market for CRISPR therapies?
- Which drug type segment accounts for maximum CRISPR therapies sales?
- What are the pricing variations among different geographies for approved CRISPR therapies?
- How has the reimbursement landscape for CRISPR therapies evolved since the first one was approved? Do patients have any access issues that are driven by reimbursement decisions?
- What are the risks, burdens, and unmet needs of treatment with CRISPR therapies? What will be the growth opportunities across the 7MM for the patient population on CRISPR therapies?
- What are the key factors hampering the growth of the CRISPR therapies market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for CRISPR therapies?
- What is the cost burden of approved CRISPR therapies on the patient?
- Patient acceptability in terms of preferred therapies options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved CRISPR therapies?
Reasons to buy
- The CRISPR MARKET report will help develop business strategies by understanding the latest trends and changing dynamics driving the CRISPR therapies Market.
- Understand the existing CRISPR market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current CRISPR patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming CRISPR companies in the CRISPR market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing CRISPR market so that the upcoming CRISPR companies can strengthen their development and launch strategy.