Breakthroughs in Alport Syndrome Treatment: A New Era of Hope
Feb 11, 2026
Table of Contents
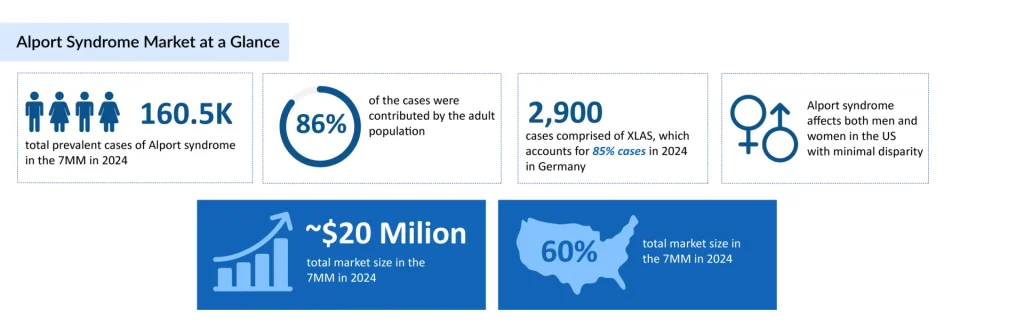
Alport syndrome, an inherited disease, predominantly manifests in its X-linked form, constituting around 80% of cases. Without intervention, roughly 90% of affected males face kidney failure by age 40, whereas females typically experience a slower progression to this condition. Many Alport syndrome cases go unnoticed due to the lack of symptoms and frequent misdiagnoses.
The total number of prevalent cases of Alport syndrome in the 7MM was nearly 160,500 in 2024 and is projected to increase by 2034, reports DelveInsight. In the US, there were nearly 13,700 diagnosed prevalent cases of Alport syndrome in 2024. According to estimates, Alport syndrome is more prevalent in the adult population, with ~86% of cases in the 7MM, whereas the pediatric population accounted for ~14%. Currently, ACE inhibitors and ARBs are the primary treatment approach for CKD in individuals with Alport syndrome. Nonetheless, recent years have seen a notable rise in efforts to develop fresh Alport syndrome new treatment options. Several established patient-initiated organizations are now dedicated to advancing and supporting clinical trials for Alport syndrome treatment. Their aim is to foster the discovery of innovative and emerging therapies for this condition.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021
- Ardelyx Overcomes Hurdles to Secure FDA Approval for Xphozah in Chronic Kidney Disease Treatment
- B. Braun’s Introcan Safety 2 IV Catheter; Everly Health’s At-Home Collection Kidney Health Test; ...
- Sky Medical’s Geko™ device; Fist Assist Devices obtains Breakthrough Device designation; E...
- ACC.25 Highlights: Groundbreaking Advances in Cardiovascular Medicine and Emerging Therapeutics
Traditional Approaches for Alport Syndrome Treatment
Treatment of Alport syndrome focuses on addressing the specific symptoms experienced by each patient. Current Alport syndrome treatment options are designed to slow disease progression, helping postpone kidney failure and extend life expectancy, although no definitive cure is available.
The primary approach of treatment for Alport syndrome involves inhibiting the renin–angiotensin–aldosterone system (RAAS) with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), as these medications lower blood pressure, reduce proteinuria, and protect renal function.
Increasing attention is also being given to sodium–glucose cotransporter-2 (SGLT2) inhibitors as potential Alport syndrome treatments to further slow the progression of chronic kidney disease (CKD). Since these drugs are already approved for their blood-glucose–lowering effects, there is growing interest in their off-label use for patients with Alport syndrome.
Emerging Therapeutic Strategies For Alport Syndrome Treatment
There is an urgent need for effective therapies for Alport syndrome treatment to address the significant burden. Companies, including Eloxx Pharmaceuticals (ELX-02), Calliditas Therapeutics (Setanaxib), Bayer/Evotec (Semaphorin-3A), ENYO Pharma [(Vonafexor (EYP001)], and others, are investigating potential drug candidates that could significantly alter the market landscape during the forecast period.
Eloxx Pharmaceuticals’ ELX-02
ELX-02 is an investigational small-molecule therapy intended to restore the production of full-length, functional proteins. In studies using immunofluorescence on fresh-frozen biopsy samples, treatment with ELX-02 has been shown to increase the levels of Collagen IV alpha5 in the glomerular basement membrane.
In April 2024, the US FDA granted ELX-02 Orphan Drug Designation for the treatment of Alport syndrome. In September 2023, Eloxx Pharmaceuticals reported independent confirmation of positive transmission electron microscopy findings from its proof-of-concept Phase II study. In that trial, all three patients who received ELX-02 showed a reduction in podocyte foot process effacement on post-treatment kidney biopsies, supporting the drug’s potential disease-modifying activity.
Following the Phase II results, the company plans to collaborate with the FDA to design a pivotal trial for ELX-02 in patients with nonsense-mutation–associated Alport syndrome and may also pursue Breakthrough Therapy Designation.
According to Aparna Thakur, Assistant Project Manager of Forecasting and Analytics at DelveInsight, ELX-02 is expected to secure a first-mover advantage in Alport syndrome and to be the first Alport syndrome gene therapy with a reduced administration frequency (8 weeks).
Calliditas Therapeutics’ Setanaxib
Setanaxib is a new dual NOX inhibitor that specifically targets the NOX4 and NOX1 enzymes, which are highly expressed in cancer-associated fibroblasts (CAFs). It is taken orally. The drug has been tested both on its own and together with ramipril, a standard ACE inhibitor. In studies, daily oral doses of 60 mg/kg setanaxib, combined with 10 mg/kg ramipril, significantly reduced fibrosis and slowed the deterioration of glomerular function. In 2023, the FDA (September) and the EMA (October) granted orphan drug designation to setanaxib for the treatment of Alport syndrome.
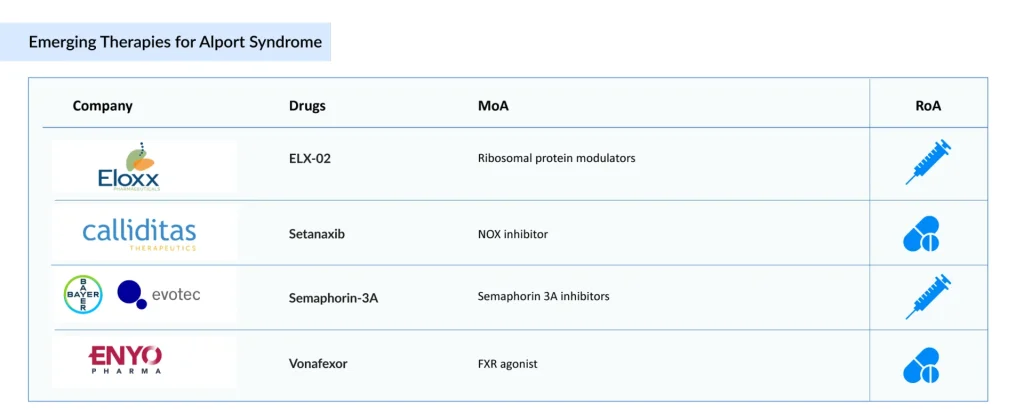
Bayer/Evotec’s Semaphorin-3A
Semaphorin-3A (Sema3A) is an extracellular signaling molecule known for its role in directing cellular movement and regulating the actin cytoskeleton. Disruptions in this cytoskeletal network, especially within podocytes, are central to the pathology of Alport syndrome, a rare inherited kidney disorder characterized by progressive loss of filtration function, eventual kidney failure, hearing decline, and sometimes visual problems. Levels of Sema3A increase in damaged human kidneys, and the protein has been linked to both the onset and worsening of various acute and chronic kidney conditions. A monoclonal antibody developed by Bayer in collaboration with Evotec that inhibits Sema3A is being studied as a potential therapy for Alport syndrome, with the goal of slowing disease progression and delaying end-stage kidney failure.
ENYO Pharma’s Vonafexor
Vonafexor is a once-daily, oral FXR agonist built on a novel, non–bile acid chemical scaffold and optimized for targeted delivery to the kidney. By modulating key metabolic, inflammatory, and fibrotic pathways, it aims to counter the fundamental mechanisms that drive renal damage and extracellular matrix changes.
In January 2026, ENYO Pharma reported positive Phase 2 Alpestria-1 results in patients with Alport syndrome. The findings showed that vonafexor, a highly differentiated FXR agonist, achieved clinically significant improvements in markers of kidney disease progression in a high-risk group already receiving standard-of-care treatment. Beyond markedly lowering albuminuria, vonafexor also appeared to reverse the typical decline in kidney function, with benefits that persisted even after therapy ended.
Introducing these promising therapies could bring significant hope to patients grappling with this condition. The advent of such molecules is expected to substantially alter the treatment paradigm for Alport syndrome.
Latest Alport Syndrome News
- In January 2026, ENYO Pharma announced that its Phase 2 Alpestria-1 trial of vonafexor in individuals with Alport syndrome yielded positive outcomes.
- In December 2025, Evotec SE (NASDAQ: EVO) announced that its partner, Bayer AG (ETR: BAYN), had initiated a Phase 2 clinical trial for a kidney-disease program arising from their joint multi-target research collaboration in kidney disorders.
- In November 2025, Calliditas Therapeutics (NASDAQ (US): CALT), a subsidiary of Asahi Kasei (TYO: 3407), reported the primary safety results from its Phase 2a randomized, double-blind, placebo-controlled study of setanaxib in Alport syndrome. The data were shared during a High-Impact Clinical Trials session at the American Society of Nephrology’s Kidney Week in Houston, Texas, on Saturday, November 8.
- In November 2025, SonoThera reported new findings at ASN Kidney Week, showing that ultrasound-mediated gene delivery can achieve full-length Col4a5 expression to treat X-linked Alport syndrome.
- In June 2025, ENYO Pharma reported that its Phase 2 ALPESTRIA-1 trial involving 26 patients with Alport syndrome was progressing as planned.
Challenges and Future Directions in Alport Syndrome Treatment
Alport syndrome presents several ongoing challenges due to its genetic complexity and progressive nature. Current therapies primarily aim to slow the progression of kidney damage, yet they cannot fully prevent the eventual decline in renal function in many patients. Variability in gene mutations across COL4A3, COL4A4, and COL4A5 leads to varying disease severity, making it difficult to design treatments that accommodate all genotypes. Early diagnosis is another major barrier; many individuals are identified only after significant kidney impairment has already occurred. Additionally, because the disease affects hearing and vision, comprehensive long-term management requires multidisciplinary care, which is not always readily accessible.
Over the next decade, improved early diagnosis and treatment advancements are expected to raise the age at which kidney failure manifests in individuals with Alport syndrome. Additional benefits are anticipated from novel therapies that can complement ACE inhibition. Safe and effective curative therapies are within the realm of possibility. As a result, the total market size of Alport syndrome is expected to increase from USD 20 million in 2024 at a significant CAGR by 2034.
Additionally, novel pharmacologic agents, including anti–microRNA therapies and drugs targeting fibrosis pathways, are also in clinical trials and may provide more tailored disease-modifying effects. Advances in early genetic screening and biomarker development could enable earlier intervention, thereby improving long-term outcomes. Ultimately, the future of Alport syndrome treatment lies in combining personalized medicine with advanced genetic therapies to move toward a curative approach.

FAQs
The total number of prevalent cases of Alport syndrome in the 7MM was nearly 160,500 in 2024 and is projected to increase by 2034, reports DelveInsight.
Key Alport syndrome therapies in the clinical trials include ELX-02, setanaxib, BAY3401016, Vonafexor, and others.
Key companies, including Eloxx Pharmaceuticals, Bayer, Calliditas Therapeutics, Evotec, ENYP Pharma, and others, are investigating their potential drug candidates that can significantly change the Alport syndrome treatment market landscape by 2034.
As per DelveInsight analysis, the total Alport syndrome market size in the 7MM was estimated to be ~USD 20 million in 2024, which is expected to show positive growth by 2034.
Downloads
Article in PDF
Recent Articles
- Baird Medical’s Microwave Ablation System; BrainSpec’s AI-Backed Solution for Non-Invasive Brain ...
- Promising Data from the First Dedicated Kidney Outcomes Trial with GLP-1 Receptor Agonist, Semagl...
- Recent Inclination of Pharma Companies, Emerging Pipeline Therapies Drive the IgA Nephropathy Mar...
- Biogen terminates ALS Pact with Karyopharm; AbbVie’s Immunological Drug Skyrizi; NICE Backs Astel...
- Assessment of Key Products that Got FDA Approval in Second Half (H2) of 2021