Targeting Multiple Sclerosis: Comparative Insights Across CD20, BTK, S1P, DHODH, and Nurr1
May 30, 2025
Table of Contents
Multiple sclerosis is a chronic, immune-mediated neurodegenerative disorder characterized by inflammation, demyelination, and gradual axonal degeneration within the central nervous system (CNS). While a range of disease-modifying therapies (DMTs) have been approved, many offer limited efficacy in slowing disease progression or preventing long-term disability, particularly in progressive forms of multiple sclerosis. Most current therapies act primarily on the peripheral immune system and have minimal impact on promoting central nervous system repair or providing neuroprotection, underscoring a significant unmet clinical need.
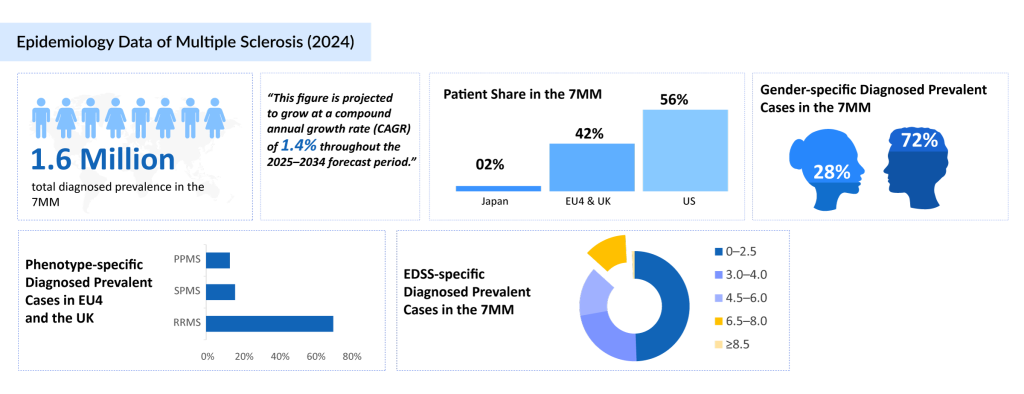
DelveInsight’s 2024 estimates indicate that approximately 1 million individuals in the United States and 700K across EU4 and the UK countries (Germany, France, Italy, Spain) and the United Kingdom were diagnosed with multiple sclerosis. In contrast, Japan reported only around 35K diagnosed cases, reflecting a significantly lower disease prevalence. This disparity is driven by several interrelated factors. Genetically, East Asian populations have a much lower frequency of the Human Leukocyte Antigen (HLA)-DRB1*15:01 allele, a key susceptibility marker common in Western populations.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Gilead Buys Out Rights to Cancer Therapy from Jounce; FDA Places Clinical Hold on Biogen’s Orelab...
- World Multiple Sclerosis Day
- Revolutionizing Multiple Sclerosis Treatment: A New Era of Therapies
- Johnson & Johnson’s TREMFYA Approved for Ulcerative Colitis; Roche’s Tecentriq Hybreza Approv...
- Late-Breaking Science at AAN 2025: Shaping the Future of Neurology
Environmental factors such as greater sun exposure and higher vitamin D levels may also contribute to a lower risk. Historically, Japan has had lower multiple sclerosis awareness, limited diagnostic capacity, and potential misclassification with conditions like neuromyelitis optica spectrum disorder (NMOSD), leading to underreporting. These insights highlight the need for more precise diagnostic frameworks and effective central nervous system-targeted treatments for both relapsing and progressive multiple sclerosis in the 7MM [the United States, EU4 (Germany, France, Italy, Spain), the United Kingdom, and Japan].
To address the substantial and growing patient pool of multiple sclerosis observed across the US, EU4 and the UK, and Japan, innovative therapeutic strategies are being developed to overcome the limitations of current disease-modifying multiple sclerosis therapies. The evolving multiple sclerosis treatment landscape features a diverse array of novel mechanisms of action (MoAs), each designed to intervene at specific molecular and cellular targets involved in multiple sclerosis pathophysiology. Selective tyrosine kinase inhibitors disrupt intracellular signaling pathways that drive immune cell activation and expansion.
Fatty acid amide hydrolase (FAAH)/monoacylglycerol lipase (MAGL) dual inhibitors reduce endocannabinoid degradation, potentially providing neuroprotection and anti-inflammatory effects within the central nervous system. Atypical Chemokine Receptor 3 (ACKR3)/ C-X-C Chemokine Receptor Type 7 (CXCR7) antagonists influence immune cell trafficking and neuroinflammation by modulating atypical chemokine receptors.
Lysophosphatidic Acid Receptor 1 (LPA1R) antagonists block lysophosphatidic acid signaling, implicated in astrocyte activation and demyelination. Tyrosine Kinase 2 (TYK2)/ Janus Kinase 1 (JAK1) dual inhibitors suppress cytokine-driven immune responses. Compounds that activate NF-E2-related factor 2 (Nrf2) enhance neuronal antioxidant defense mechanisms. Additionally, muscarinic M1 receptor antagonists regulate cholinergic pathways, offering possible roles in immune modulation and remyelination.
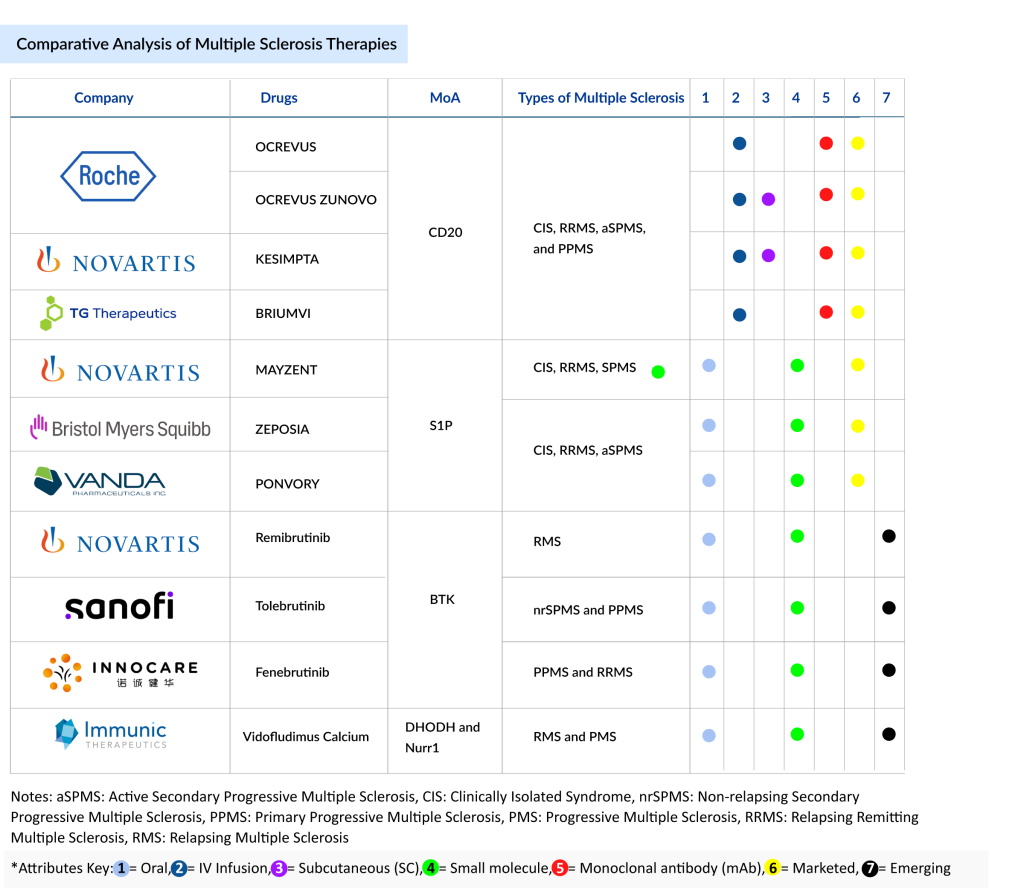
Despite this mechanistic diversity, the current and emerging multiple sclerosis market is primarily driven by some major mechanisms of action: CD20-targeting monoclonal antibody, Bruton’s Tyrosine Kinase (BTK) inhibitors, Sphingosine-1-Phosphate Receptor (S1PR) modulators, Dihydroorotate Dehydrogenase (DHODH) inhibitors, and Nurr1 agonists. CD20 and sphingosine-1-phosphate receptor modulators are well-established, representing the backbone of current multiple sclerosis therapy. Meanwhile, Bruton’s Tyrosine Kinase and Dihydroorotate Dehydrogenase inhibitors, along with Nurr1 agonists, represent promising emerging modalities due to their potential to address both inflammatory and neurodegenerative components of multiple sclerosis.
CD20 Monoclonal Antibodies (CD20 mAbs) in Multiple Sclerosis Treatment
CD20-targeting monoclonal antibodies (mAbs) have become foundational in the treatment of multiple sclerosis, particularly for relapsing forms and primary progressive multiple sclerosis. Approved multiple sclerosis drugs include OCREVUS (ocrelizumab), OCREVUS ZUNOVO (ocrelizumab and hyaluronidase-ocsq), KESIMPTA (ofatumumab), and BRIUMVI (ublituximab-xiiy). OCREVUS and its subcutaneous (SC) formulation, OCREVUS ZUNOVO, powered by Halozyme’s Enhanze drug delivery technology, are uniquely approved for Clinically Isolated Syndrome (CIS), Relapsing Remitting Multiple Sclerosis (RRMS), Active Secondary Progressive Multiple Sclerosis (aSPMS), and Primary Progressive Multiple Sclerosis, making them the only agents approved for Primary Progressive Multiple Sclerosis (PPMS) to date.
At the American Academy of Neurology (AAN) 2025, results from the OCARINA II study highlighted the subcutaneous formulation of ocrelizumab as a well-tolerated, effective treatment for relapsing and primary progressive multiple sclerosis. Over 72 weeks, subcutaneous ocrelizumab maintained clinical efficacy with near-complete suppression of relapse activity and stable disability scores. Injection reactions were mostly mild or moderate and declined over time. No new safety concerns or treatment-emergent anti-drug antibodies to ocrelizumab were observed. Most patients reported high satisfaction, with 80% preferring subcutaneous over intravenous administration. The subcutaneous formulation offers a faster (10-minute) alternative without compromising the established benefit–risk profile of intravenous ocrelizumab. The company plans to file for Relapsing Multiple Sclerosis (RMS) and primary progressive multiple sclerosis adult indications in 2025.
KESIMPTA, indicated for clinically isolated syndrome, relapsing remitting multiple sclerosis, and active secondary progressive multiple sclerosis, demonstrated sustained efficacy and safety in the ALITHIOS open-label extension, with 93% of early multiple sclerosis patients achieving No Evidence of Disease Activity (NEDA-3) status by Year 6. These results, presented at the American Academy of Neurology (AAN) 2025 Annual Meeting, highlighted significant reductions in disease progression and serum neurofilament light levels. KESIMPTA is also being studied in Phase III clinical trials, including a pediatric MS trial with a planned regulatory submission in 2027, as well as a separate study on a new dosing regimen expected to be filed in 2028 or beyond.
BRIUMVI, approved for relapsing multiple sclerosis subtypes, showed favorable tolerability in real-world settings per the ENAMOR survey, also presented at the American Academy of Neurology (AAN) 2025. Lower rates of infusion-related reactions compared to pivotal trials may be linked to real-world premedication practices. The ENABLE Phase IV study will further explore long-term outcomes. Collectively, CD20 therapies remain a dominant and evolving pillar of multiple sclerosis treatment.
Precision Oral Multiple Sclerosis Treatment Drugs on the Rise
Bruton’s Tyrosine Kinase inhibitors are emerging as a promising new class of oral small-molecule multiple sclerosis drugs poised to reshape the multiple sclerosis treatment paradigm. These agents offer a unique mechanism by targeting both peripheral B cells and central nervous system-resident microglia, addressing inflammation and neurodegeneration more comprehensively than current therapies. Several Bruton’s Tyrosine Kinase inhibitors are advancing through late-stage clinical development. Novartis’s remibrutinib (LOU064) is in Phase III trials for adults with relapsing multiple sclerosis.
Sanofi’s tolebrutinib (SAR442168), with FDA Priority Review and Breakthrough Therapy Designation (BTD), is being evaluated across relapsing multiple sclerosis, primary progressive multiple sclerosis, and non-relapsing Secondary Progressive multiple sclerosis, reflecting its broad therapeutic potential. At the American Academy of Neurology (AAN) 2025, Sanofi presented key findings on tolebrutinib, an oral Bruton’s tyrosine kinase inhibitor under FDA Priority Review and breakthrough therapy designation for multiple sclerosis treatment.
The HERCULES Phase III trial demonstrated a significant 31% reduction in 6-month Confirmed Disability Progression (CDP) in on-relapsing secondary progressive multiple sclerosis. Although the GEMINI 1 and 2 studies in relapsing multiple sclerosis showed no superiority over teriflunomide in reducing relapse rates, tolebrutinib delayed disability worsening by 29%. Safety profiles were generally balanced, with manageable liver enzyme elevations. These data underscore tolebrutinib’s potential to address chronic central nervous system inflammation and disability in diverse multiple sclerosis populations.
Roche’s fenebrutinib is also in Phase III for both primary progressive multiple sclerosis and relapsing multiple sclerosis; at the American Academy of Neurology (AAN) 2025 Annual Meeting, Roche presented updated data from the FENopta open-label extension study, demonstrating that fenebrutinib, an oral, reversible, central nervous system-penetrant Bruton’s tyrosine kinase inhibitor, sustained low disease activity in patients with relapsing multiple sclerosis through 48 weeks. Impressively, 89.6% of participants achieved NEDA-3 status, defined as the absence of relapses, Magnetic resonance imaging (MRI) activity, and 24-week confirmed disability progression.
Additionally, 96% of patients remained relapse-free, while 99% had no new T1 gadolinium-enhancing (T1 Gd+) lesions at Week 48, and median Expanded Disability Status Scale (EDSS) scores remained stable. Safety outcomes were favorable, with most AEs mild to moderate and no new safety signals reported. Only one serious SAE (nephrolithiasis and UTI) and one Grade 3 ALT elevation were noted, resolving with treatment discontinuation.
These findings reinforce fenebrutinib’s potential as a disease-modifying multiple sclerosis medication that effectively targets both peripheral and compartmentalized central nervous system inflammation, while InnoCare’s orelabrutinib and Biogen’s BIIB091 are in Phase II multiple sclerosis clinical trials targeting progressive and relapsing multiple sclerosis forms.
InnoCare’s orelabrutinib (ICP-022), an oral, small-molecule Bruton’s tyrosine kinase inhibitor, is advancing in clinical development for the treatment of progressive and relapsing forms of multiple sclerosis. Following a successful End-of-Phase II meeting with the US FDA, InnoCare has received clearance to initiate a Phase III trial targeting primary progressive multiple sclerosis, with enrollment expected to begin by mid-2025.
The US FDA also encouraged the company to pursue a second pivotal study in secondary progressive multiple sclerosis, with plans to enroll the first patient by the end of 2025. At the 2025 Americas Committee for Treatment and Research in Multiple Sclerosis Forum, InnoCare presented compelling Phase II results in relapsing multiple sclerosis, where the 80 mg once-daily dose achieved significant reductions in gadolinium-enhancing (Gd+) lesions, 90.4% at week 12 and 92.3% at week 24, vs. placebo and lower doses. Lesion suppression was evident by Week 4 and remained durable through Week 24. The treatment was well tolerated across all dosing groups, with no serious Treatment-Emergent Adverse Events (TEAEs) observed in the 80 mg cohort. These results position orelabrutinib as a strong candidate among emerging Bruton’s tyrosine kinase inhibitors for both relapsing multiple sclerosis and progressive multiple sclerosis.
The sphingosine-1-phosphate receptor modulators represent a key class of oral therapies in the treatment of multiple sclerosis, particularly for relapsing forms and active secondary progressive multiple sclerosis. Among these, MAYZENT (siponimod), developed by Novartis, holds regulatory approval in the US for the clinically isolated syndrome, relapsing remitting multiple sclerosis, and active secondary progressive multiple sclerosis, while its label is more restricted to active secondary progressive multiple sclerosis in the European Union (EU) and secondary progressive multiple sclerosis in Japan. Approved between 2019 and 2020 across key global markets, MAYZENT stands out as the first sphingosine-1-phosphate receptor modulator specifically approved for secondary progressive multiple sclerosis with active disease.
ZEPOSIA (ozanimod), developed by Bristol Myers Squibb, gained approval in 2020 in both the US and EU for clinically isolated syndrome, relapsing remitting multiple sclerosis, and active secondary progressive multiple sclerosis, expanding its utility as a broad-spectrum sphingosine-1-phosphate receptor therapy with a favorable safety and cardiac profile. At the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) 2025, interim findings from the ongoing Phase III multiple sclerosis clinical trial (ENLIGHTEN study) highlighted neurofilament light chain as a potential biomarker of treatment response in early relapsing multiple sclerosis patients receiving ZEPOSIA.
After 1 year of treatment, mean neurofilament light chain levels decreased from 14.6 pg/mL to 9.3 pg/mL. Lower neurofilament light chain (NfL) concentrations correlated with better cognitive performance, as shown by improvements in SDMT, CVLT-II, and BVMT-R scores, and with favorable magnetic resonance imaging outcomes, including fewer and smaller lesions and greater preservation of brain volumes. These results support neurofilament light chain’s utility in monitoring neurodegeneration and therapeutic efficacy in relapsing multiple sclerosis.
PONVORY (ponesimod), originally developed by Janssen and now commercialized by Vanda and Juvisé, was approved in 2021 in the US and EU for clinically isolated syndrome, relapsing remitting multiple sclerosis, and active secondary progressive multiple sclerosis. It is distinguished by its rapid clearance and flexible dosing, which may offer clinical advantages in patient management. Collectively, these agents reinforce the therapeutic value of sphingosine-1-phosphate receptor modulation in managing inflammatory activity and disease progression in multiple sclerosis.
Vidofludimus calcium (IMU-838), developed by Immunic Therapeutics, is a novel, orally administered small molecule currently in Phase III trials for relapsing multiple sclerosis. It employs a unique dual mechanism of action: Nurr1 activation, which delivers direct neuroprotective benefits, and Dihydroorotate Dehydrogenase inhibition, which provides anti-inflammatory effects.
This first-in-class approach aims to address both the inflammatory and neurodegenerative components of multiple sclerosis, offering potential therapeutic benefits across the disease spectrum, including relapsing and progressive forms. Phase II studies have demonstrated efficacy in relapsing-remitting multiple sclerosis, progressive multiple sclerosis, and ulcerative colitis. If successful, vidofludimus calcium could become the first oral disease-modifying therapy approved for both relapsing and progressive multiple sclerosis.
From Infusion to Ingestion: Multiple Sclerosis Treatment Shifts Toward Oral Drugs
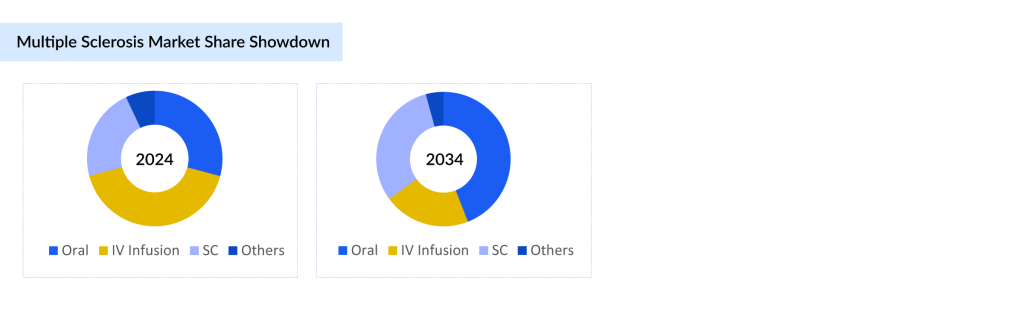
The multiple sclerosis treatment landscape is undergoing a profound shift, with oral therapies rapidly emerging as the preferred route of administration. According to DelveInsight estimates, oral multiple sclerosis treatment drugs are projected to increase their market share from 29% in 2024 to 44% by 2034 in the 7MM, outpacing all other delivery methods. This significant rise reflects a broader movement toward patient-friendly treatments, driven by improved adherence, greater convenience, and the growing availability of effective oral disease-modifying therapies, including those currently in late-stage development.
In contrast, IV infusion, which held the largest multiple sclerosis market share at 42% in 2024, is expected to decline markedly to 21% by 2034. Subcutaneous administration is anticipated to grow steadily, increasing from 22% to 31% over the same period. Meanwhile, all other administration routes, including intramuscular and combined subcutaneous/intramuscular methods are grouped as “Others” and are projected to decrease from 7% in 2024 to just 4% by 2034.
These insights, based on DelveInsight’s in-depth market analysis incorporating revenues from both current therapies and late-stage multiple sclerosis pipeline candidates, highlight a clear trend. The future of multiple sclerosis treatment is being shaped by a strong shift toward noninvasive, accessible options, placing oral multiple sclerosis drugs at the forefront of therapeutic and commercial strategies.
Future of Multiple Sclerosis Treatment
The multiple sclerosis treatment paradigm is shifting from primarily targeting peripheral immune modulation toward comprehensive strategies that address both inflammation and neurodegeneration. While CD20 monoclonal antibodies remain foundational, the emergence of Bruton’s Tyrosine Kinase inhibitors, sphingosine-1-phosphate receptor modulators, dihydroorotate dehydrogenase inhibitors, and first-in-class Nurr1 agonists highlights a growing mechanistic diversification aimed at broader and more effective disease control.
A strong multiple sclerosis pipeline of oral therapies, especially those with central nervous system-penetrant and neuroprotective properties, is driving a marked transition in route of administration preferences, with oral disease-modifying multiple sclerosis drugs expected to become the dominant modality by 2034. This evolution not only reflects therapeutic innovation but also improved patient adherence and convenience.
As precision oral therapies increasingly demonstrate efficacy in both relapsing and progressive multiple sclerosis, future prospects include higher treatment penetration and a potential disease-modifying impact in forms previously considered refractory. These new multiple sclerosis treatments are poised to enhance the overall treatment rate of multiple sclerosis by making effective options more accessible and tolerable.
Additionally, the integration of biomarker-driven approaches, such as neurofilament light chain monitoring, and adaptive clinical trial designs will further refine therapeutic targeting and optimize outcomes. Collectively, these trends position multiple sclerosis care for transformative advances in the coming decade.

Downloads
Article in PDF
Recent Articles
- Late-Breaking Science at AAN 2025: Shaping the Future of Neurology
- Allumiqs and Prolytix’s Partnership; Setpoint Medical’s Neuroimmune Modulation Platform get...
- Celiac Disease – Intestinal damage of Gluten
- Virus, the Cancer Therapy of the Future
- BMS, and Exelixis’s Opdivo + CABOMETYX in First-Line Advanced Renal Cell Carcinoma; AIRSUPRA Now ...