Vanda Broadens its Reach to Bipolar Disorder Treatment Market With Fanapt Approval
Apr 05, 2024
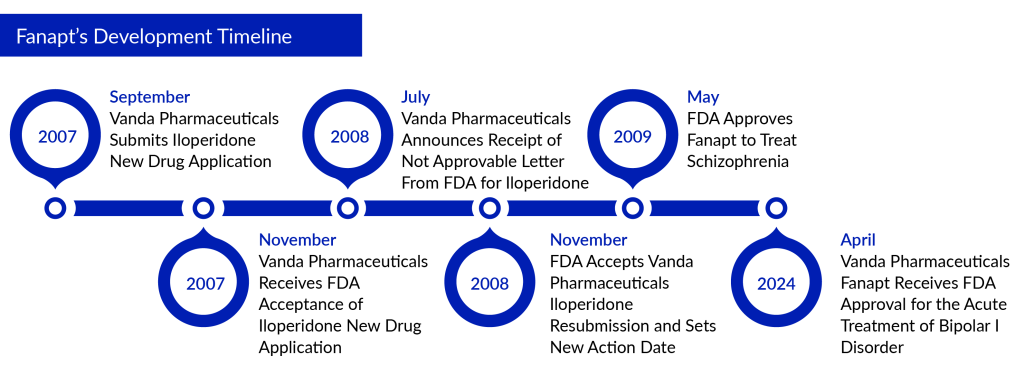
Fifteen years after its first FDA approval for treating schizophrenia with Fanapt (iloperidone), Vanda Pharmaceuticals has achieved another success. Fanapt is a type of antipsychotic medication that deviates from the usual and has been administered to individuals with schizophrenia for their immediate treatment following its approval by the FDA in 2009. Despite its label stating that the precise method of operation remains unidentified, Vanda suggests that Fanapt functions by inhibiting dopamine type 2 and serotonin type 2 receptors. The FDA has approved Fanapt tablets for the treatment of manic or mixed episodes linked to bipolar I disorder in adults. This led to a 33% surge in Vanda’s shares during post-market trading.
Affecting approximately 2.8% of adults in the United States, bipolar disorder is a significant mental health condition distinguished by episodes of low mood interspersed with episodes of elevated mood. In addition, the total bipolar I disorder cases of bipolar depression in the US were observed as ~935K in the year 2021, these cases are estimated to increase in upcoming years. According to our analysis, ~75% of bipolar depression cases were classified as severe in the US in 2021.

“Manic or mixed episodes linked with bipolar I disorder are intricate conditions that demand a range of reliable choices to address the unique needs of each patient. With more than 100,000 patient years of usage, Fanapt is a recognized treatment option that allows for adaptable dosing while maintaining a well-established safety record. This approval from the FDA provides patients and healthcare providers with a fresh approach to handling bipolar I disorder,” stated Mihael H. Polymeropoulos M.D., who serves as Vanda’s President, CEO, and Chairman of the Board.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Plotting the Extensive Demand of Mobile Apps for Mental Health
- FDA Approves Luye’s Rykindo; EU Approves AstraZeneca’s Tezspire; Oramed Announces Trial Results o...
- Aminex Therapeutics Secures FDA Orphan Drug Designation for AMXT 1501 + DFMO in Neuroblastoma; Ec...
- Neuroscience Highlights – 17/07/2018
- Hope on the Horizon: Brilaroxazine’s Promise for Schizophrenia Patients
Dr. Polymeropoulos added, “The announcement made today represents a notable progression for one of Vanda’s key franchises, highlighting the success of our approach in developing groundbreaking treatments for important medical challenges, ultimately enhancing the well-being of patients. Building upon this achievement, we have built a robust business with a variety of upcoming products, a track record of increasing revenue, and solid financial standing. Our primary commitment remains delivering essential medications to patients globally, all the while fostering enduring, sustainable value.”
The FDA’s approval is supported by information gathered from a Phase III clinical study involving approximately 400 individuals diagnosed with bipolar I disorder. These participants were in the midst of experiencing a manic episode at the time of the trial. Following a four-week treatment period, patients who received Fanapt exhibited notably more substantial enhancements according to the Young Mania Rating Scale, a measure used to evaluate the intensity of mania, when compared to those who received a placebo. Furthermore, the study revealed that Fanapt has the potential to provide swift benefits for bipolar I disorder, as its significant superiority over the placebo became evident as early as two weeks into the treatment.
Fanapt includes a boxed warning indicating a higher potential for mortality in elderly patients with dementia-related psychosis when using antipsychotic medications. Vanda is also exploring the use of Fanapt as a remedy for post-traumatic stress disorder (PTSD).
Iloperidone has an extensive background dating back to the mid-1990s when it was under development by Novartis and Titan Pharmaceuticals until Vanda acquired it in 2004. Despite initially rejecting iloperidone in 2008 due to insufficient clinical data, the FDA approved it in 2009.
When it was introduced, several analysts predicted that sales could hit $300 million by 2013. However, the drug fell short of these projections. Throughout the following ten years, sales grew gradually, reaching a high point of $95 million in 2021. Last year, Fanapt brought in $90 million in revenue.
This successful regulatory outcome will assist Vanda in recovering following the FDA’s recent denial of its insomnia treatment, Hetlioz (tasimelteon), which targets melatonin receptors. The rejection follows a prior communication from the regulatory body, indicating issues that currently prevent the discussion of labeling and post-marketing obligations.
Vanda’s decrease in revenue during the past couple of years is mainly because of the dropping sales of its other key product, Hetlioz (tasimelteon). The rise of generic alternatives in the US has led to a significant decline in Hetlioz sales, plummeting from $160 million in 2022 to $100 million in the previous year. Additionally, Vanda has faced repeated setbacks in its attempts to secure approvals for Hetlioz’s use in treating insomnia and jet lag disorder.
The company aims to offset the loss in revenue by investing $100 million to acquire rights for Johnson & Johnson’s Ponvory, a treatment for multiple sclerosis, in the US and Canada. Vanda finalized this agreement in December of the previous year.
However, Vanda will get fierce competition from other companies working with their lead assets to improve the bipolar disorder treatment space. One such company working with its asset in the late stage of development is Lyndra Therapeutics (LYN-005).
LYN-005, an oral weekly risperidone, is in development for individuals with schizophrenia and bipolar I disorder. Utilizing Lyndra’s innovative LYNX drug delivery system, this medication offers a once-a-week dosage option compared to the daily regimen of risperidone.
The LYNX platform, stemming from innovations in the Langer Lab, has swiftly advanced since 2015, traversing preclinical and initial human trials. It has validated the platform’s concept and primary asset through a Phase II study recently completed. With its versatility spanning various therapeutic domains, including both existing and emerging drugs, the LYNX drug delivery system holds promise for enhancing treatment adherence and health outcomes. Additionally, it offers the prospect of liberating individuals from the constraints of daily pill regimens, thereby streamlining their lives.
Phase II findings for oral weekly risperidone showed rapid attainment of therapeutic levels, sustained drug levels for weekly dosing, and decreased peak drug exposure. This oral weekly formulation has the potential to become the initial long-lasting oral treatment for individuals managing schizophrenia and bipolar I disorder.
In April 2023, Lyndra Inc. began a study with multiple doses, open-label, parallel-group design to assess the pharmacokinetics, safety, and tolerability of their long-acting oral Risperidone (LYN-005) in individuals diagnosed with Schizophrenia or Schizoaffective Disorder.
The pivotal study included participants with schizophrenia and schizoaffective disorder who were on a stable dose of an oral antipsychotic medication at the start of the study. The study assessed oral weekly risperidone 15 mg and 45 mg, equivalent to 2 mg and 6 mg daily Risperdal, respectively. A total of 90 participants were planned for the study, with a planned interim analysis after 46 participants.
In the interim analysis, the study met the pre-specified primary endpoint, which includes the geometric mean ratio of the Week 5 oral weekly risperidone drug levels compared to the baseline immediate-release Risperdal drug levels. Oral weekly risperidone met the endpoint of a one-sided Cmin of >0.80; a one-sided Cmax of <1.25; and a two-sided Cavg of 0.80-1.40, with 90% confidence limits. With these positive results, the study was stopped early according to the pre-defined stopping criteria.
The study also met its secondary endpoints for safety and the Positive and Negative Syndrome Scale (PANSS) score, a score used for measuring symptom severity in schizophrenia. One key potential benefit of the LYNX drug delivery platform is the ability to deliver more consistent levels of medication compared to daily dosing by reducing the peak-to-trough variation. Oral weekly risperidone (LYN-005) was generally safe and well tolerated.
The approval of LYN-005 will be a great threat to Vanda’s Fanapt. As of now, Vanda Pharmaceuticals’ Fanapt has long been a cornerstone in the treatment of schizophrenia. Its effectiveness and the trust it has garnered among physicians and patients alike have contributed significantly to Vanda’s position in the pharmaceutical market. However, the landscape might shift with the potential approval of Lyndra’s LYN-005 for the treatment of bipolar disorder, posing challenges and raising questions about Fanapt’s future sales trajectory.
One key aspect that might impact Fanapt’s sales is the efficacy and safety profile of LYN-005. If the new drug demonstrates superior efficacy, fewer side effects, or a more convenient dosing regimen, physicians and patients could be swayed to switch from Fanapt to the new option. This scenario could lead to a gradual decline in Fanapt’s market share, especially if LYN-005 gains momentum post-approval.
Vanda will need to navigate this evolving landscape strategically, focusing on differentiation, innovation, and effective communication to maintain Fanapt’s position in the bipolar disorder market amidst emerging competition.

Downloads
Article in PDF
Recent Articles
- Breaking Boundaries: Innovations and Updates in Schizophrenia Treatment
- Evenamide (NW-3509): Advancing Solutions for Treatment-Resistant Schizophrenia
- Hope on the Horizon: Brilaroxazine’s Promise for Schizophrenia Patients
- FDA Approves Luye’s Rykindo; EU Approves AstraZeneca’s Tezspire; Oramed Announces Trial Results o...
- Fewer Sleepless Nights Over, Drugs for Insomnia Treatment