Expanding Horizons in Chronic Pruritus Treatment: Emerging Therapies Target Diverse Mechanisms
Oct 13, 2025
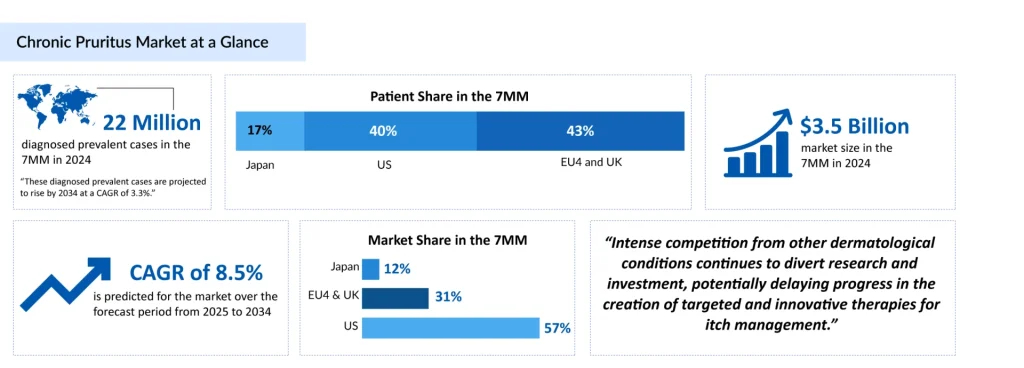
Chronic pruritus, characterized by itching that persists for more than six weeks, is a multifaceted condition that may stem from dermatologic, systemic, neurologic, or psychogenic factors. It has a profound impact on patients’ quality of life and is increasingly recognized as a standalone clinical entity. DelveInsight estimates that there were approximately 98 million prevalent cases of chronic pruritus and 22 million diagnosed prevalent cases across the 7MM in 2024. These diagnosed prevalent cases are projected to rise by 2034 at a CAGR of 3.3%.
Managing this condition effectively involves determining its root cause and implementing targeted treatments. As the understanding of itch mechanisms advances, therapy is progressively moving toward precision medicine, incorporating innovative agents designed to address the condition’s complex pathophysiology.
Chronic Pruritus: A Market with Sparse Therapeutic Options
Current treatment strategies for chronic pruritus encompass a range of options, including steroids, antihistamines, calcineurin inhibitors (such as cyclosporine and tacrolimus), gabapentin, pregabalin, serlopitant, fluoxetine, sertraline, nalfurafine hydrochloride, kappa opioid receptor agonists, and IBAT inhibitors.
Downloads
Click Here To Get the Article in PDF
Steroids and antihistamines are commonly used for broad symptom management, though their efficacy can vary depending on the underlying cause. Calcineurin inhibitors, including cyclosporine and tacrolimus, help regulate immune-mediated itch, particularly in dermatologic conditions. Other agents, such as gabapentin, pregabalin, serlopitant, fluoxetine, sertraline, and nalfurafine hydrochloride, act on neural or serotonergic pathways, providing relief in neuropathic or treatment-resistant pruritus when conventional therapies are insufficient.
The chronic pruritus market is currently not highly competitive, with only a few chronic pruritus drugs approved for treatment, including KORSUVA/KAPRUVIA (Cara Therapeutics/CSL/ Vifor/Maruishi), LIVMARLI (Mirum Pharmaceuticals/Takeda), and BYLVAY/KAYFANDA (Mirum Pharmaceuticals/Takeda).
Difelikefalin, a kappa opioid receptor agonist, is approved in the US as KORSUVA and in the EU and UK as KAPRUVIA for the treatment of moderate-to-severe pruritus in adults with chronic kidney disease undergoing hemodialysis. Though its exact mechanism is not fully understood, it is thought to modulate itch signaling in the central and peripheral nervous systems.
In August 2021, the US FDA approved KORSUVA injection from Cara Therapeutics and Vifor Pharma, marking it as the first therapy for moderate-to-severe CKD-aP. Following this, in April 2022, KAPRUVIA received EU-wide marketing authorization for the treatment of moderate-to-severe CKD-aP in adults undergoing hemodialysis. In September 2023, Maruishi received approval in Japan for the KORSUVA IV injection syringe for treating pruritus in hemodialysis patients.
LIVMARLI (maralixibat), an IBAT inhibitor, is approved for treating cholestatic pruritus in patients with Alagille Syndrome (ALGS) aged 3 months and older, and Progressive Familial Intrahepatic Cholestasis (PFIC) aged 12 months and older. It reduces bile acid reabsorption from the terminal ileum, though the exact mechanism for alleviating pruritus remains unclear. Maralixibat is also being evaluated in a Phase III trial for pruritus in cholestatic liver disease.
In March 2025, it became the first and only approved treatment for ALGS and PFIC in Japan. In March 2024, the US FDA approved it for PFIC. Earlier, in March 2023, the US FDA expanded its label for ALGS treatment in patients aged three months and older. The EC approved it for PFIC in July 2024 and for ALGS in December 2022. It was first approved by the US FDA in September 2021 for ALGS in patients aged one year and older.
BYLVAY/KAYFANDA (odevixibat), an IBAT inhibitor, is approved for treating pruritus in patients aged 3 months and older with PFIC and aged 12 months and older with ALGS. It works by reducing bile salt reabsorption from the terminal ileum. While its exact mechanism in relieving pruritus is unclear, it is believed to involve reduced bile salt reuptake, lowering serum bile acid levels. BYLVAY may not be effective in some PFIC type II patients with specific ABCB11 gene variants. It is also being evaluated in a Phase III trial for biliary atresia, with results expected in 2026.
In September 2024, Ipsen’s KAYFANDA was approved by the European Union for treating cholestatic pruritus in ALGS patients aged 6 months or older. In June 2023, the US FDA approved BYLVAY for cholestatic pruritus in patients aged 12 months and older with ALGS, following its approval for PFIC-related pruritus in July 2021. In July 2021, BYLVAY also received EU-wide marketing authorization for treating patients aged 6 months and older with PFIC.
Promising Candidates in Development for Chronic Pruritus Treatment
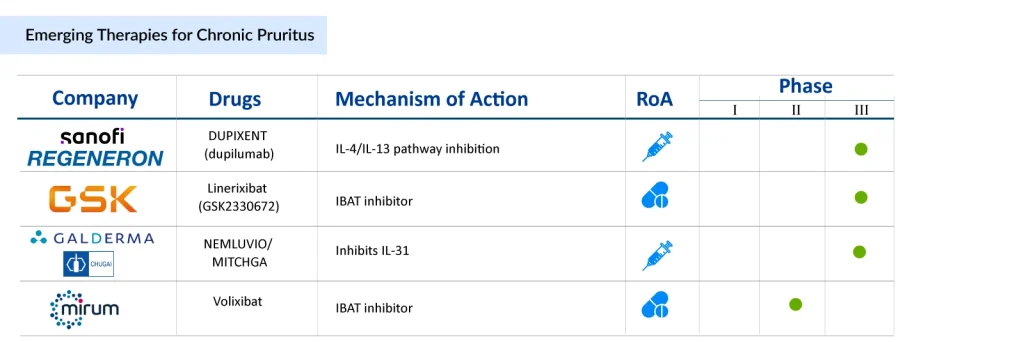
The chronic pruritus treatment landscape is witnessing a notable expansion, driven by a deeper understanding of the condition’s multifactorial mechanisms. Several promising candidates are advancing through clinical development, targeting diverse pathways to address both symptom relief and underlying pathophysiology. Biologics such as dupilumab (Sanofi/Regeneron), which modulate type 2 inflammation, continue to demonstrate robust efficacy in patients with atopic dermatitis-associated pruritus. At the same time, novel chronic pruritus drugs like nemolizumab (Galderma/Chugai Pharmaceutical) target the IL-31 receptor, offering a potentially transformative option for refractory cases. In parallel, emerging small-molecule therapies, including IBAT inhibitors like linerixibat (GSK) and volixibat (Mirum Pharmaceuticals), are exploring non-immune-mediated mechanisms, broadening therapeutic options for patients with cholestatic or systemic pruritus.
This diversification reflects growing recognition of chronic pruritus as a condition with significant unmet need and commercial potential. The pipeline’s breadth, from receptor-specific biologics to pathway-targeted small molecules, underscores both scientific innovation and the strategic focus of developers on differentiated mechanisms. As these candidates progress through late-stage trials, their ability to demonstrate meaningful, durable relief with favorable safety profiles will be critical for market adoption. Analysts will be closely monitoring regulatory milestones, head-to-head efficacy data, and potential combination strategies, as these factors are likely to shape competitive positioning and long-term revenue trajectories in this evolving therapeutic segment.
Chronic Pruritus Market Outlook Looks Promising
The chronic pruritus market is poised for significant growth from USD 3.5 billion in 2024 across the major markets at a CAGR of 8.5% by 2034. The anticipated increase in market size is driven by advancements in treatment options, greater healthcare access, and a rising prevalence of the condition, which together foster higher demand for innovative and effective therapies.
Emerging treatments such as dupilumab, nemolizumab, linerixibat, and volixibat are addressing distinct molecular pathways, ranging from cytokine modulation to bile acid transport inhibition. This diversification not only expands the therapeutic arsenal but also highlights the market’s shift from symptom management toward mechanism-based interventions, offering hope for improved patient outcomes across dermatologic and systemic conditions associated with chronic itch.The chronic pruritus market is benefiting from heightened awareness, increasing prevalence in aging populations, and a growing emphasis on quality-of-life improvement. Payers and clinicians are increasingly receptive to innovative therapies that demonstrate both efficacy and tolerability, which is expected to drive adoption and support premium pricing strategies. Combined with ongoing chronic pruritus clinical trials and pipeline expansion, the market outlook remains robust, positioning chronic pruritus as an attractive segment for investment and strategic partnerships in the coming decade.

Downloads
Article in PDF