4 Investigational Chronic Pruritus Drugs Shaping the Treatment Landscape
Oct 24, 2025
The chronic pruritus drugs market is currently not highly competitive, with only a few chronic pruritus drugs approved for treatment, including KORSUVA/KAPRUVIA (Cara Therapeutics/CSL/ Vifor/Maruishi), LIVMARLI (Mirum Pharmaceuticals/Takeda), and BYLVAY/KAYFANDA (Mirum Pharmaceuticals/Takeda). However, the treatment landscape for chronic pruritus is rapidly evolving, fueled by growing insights into the condition’s complex and multifactorial nature.
A range of promising chronic pruritus drug candidates is advancing through clinical trials, each designed to target distinct biological pathways to alleviate symptoms and address the root causes. Biologics such as dupilumab (Sanofi/Regeneron), which act on type 2 inflammatory signaling, have shown strong efficacy in managing atopic dermatitis-related itch. Meanwhile, innovative agents like nemolizumab (Galderma/Chugai Pharmaceutical), an IL-31 receptor antagonist, present a potential breakthrough for patients with difficult-to-treat pruritus. Additionally, new small-molecule therapies, such as IBAT inhibitors linerixibat (GSK) and volixibat (Mirum Pharmaceuticals), are being developed to tackle non-immune mechanisms, expanding treatment options for those with cholestatic or systemic forms of the disease.
Now, let’s dive deep into the detailed assessment of these four promising chronic pruritus drugs under development.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- DUPIXENT Receives First-Ever Biologic Approval for COPD: Adds Another Jewel in its Crown
- Sanifit nets USD 80.9 M; FDA approves Dupixent for CRS; AbbVie buys Allergan
- DUPIXENT’s Launch Brings Next Chapter of Biologics in COPD
- How Emerging Pipeline Therapies Will Unfold the Severe Asthma Treatment Market Dynamics?
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
Sanofi/Regeneron’s DUPIXENT
DUPIXENT (dupilumab) is a non-immunosuppressive monoclonal antibody that inhibits IL-4 and IL-13 signaling, key mediators of Type 2 inflammation. The drug is in Phase III clinical development for chronic pruritus of unknown origin (CPUO), aiming to provide relief for patients suffering from persistent itching with few available treatments by targeting the underlying inflammatory mechanisms.
In September 2024, results from the Phase III LIBERTY-CPUO-CHIC trial in adults with uncontrolled, severe CPUO did not achieve statistical significance for the primary itch-responder endpoint, though favorable numerical trends were observed. The study demonstrated nominally significant improvements across all other itch-related secondary endpoints. The Phase III program consists of Study A (LIBERTY-CPUO-CHIC) and the planned pivotal Study B.
GlaxoSmithKline’s Linerixibat
Linerixibat is an oral ileal bile acid transporter (IBAT) inhibitor in development for the treatment of cholestatic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disorder. By preventing the reuptake of bile acids, it targets a key mechanism driving pruritus. The US FDA granted Orphan Drug Designation to linerixibat in 2019 for PBC and related cholestatic pruritus. In June 2025, the FDA accepted the New Drug Application (NDA) for linerixibat, setting a PDUFA target date of March 24, 2026. The therapy is also being investigated in an ongoing long-term Phase III study to evaluate its safety and tolerability in PBC patients.
In May 2025, results from the Phase III GLISTEN trial demonstrated that linerixibat achieved its primary endpoints, producing rapid and sustained improvements in cholestatic pruritus and associated sleep disturbances in individuals with PBC.
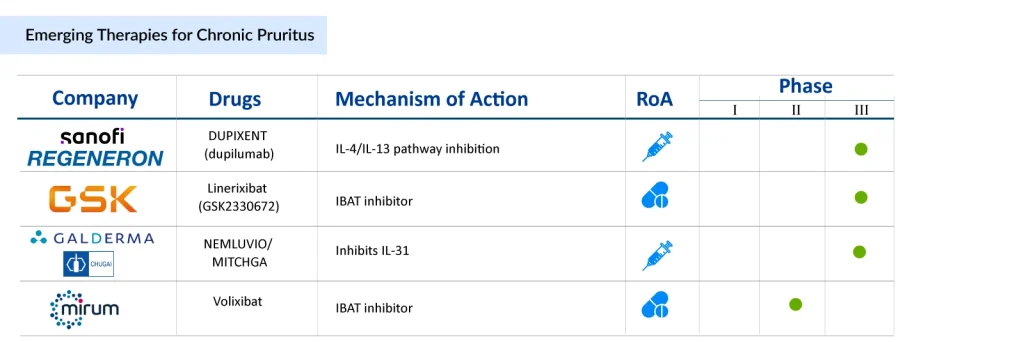
Galderma/Chugai Pharmaceutical’s NEMLUVIO/MITCHGA
Nemolizumab, a first-in-class humanized monoclonal antibody targeting the interleukin-31 receptor A (IL-31RA), directly modulates the neuroimmune signaling responsible for chronic itch. In August 2024, the FDA approved nemolizumab for the treatment of adults with prurigo nodularis. Later, in December 2024, the FDA also approved it for patients aged 12 years and older with moderate-to-severe atopic dermatitis, to be used alongside topical corticosteroids and/or calcineurin inhibitors when standard topical prescription therapies are insufficient.
Currently, nemolizumab has received approval for both moderate-to-severe atopic dermatitis and prurigo nodularis from multiple regulatory agencies worldwide, including those in the European Union, Australia, Singapore, Switzerland, and the United Kingdom, with further submissions and reviews ongoing.
Developed initially by Chugai Pharmaceutical Co., Ltd., nemolizumab’s global development and marketing rights (excluding Japan) were acquired by Galderma in 2016. In Japan, it is sold under the brand name MITCHGA. It is approved for treating prurigo nodularis as well as pruritus associated with atopic dermatitis in pediatric, adolescent, and adult patients.
In June 2025, Galderma announced the launch of new clinical trials to evaluate the efficacy and safety of nemolizumab for patients with Chronic Pruritus of Unknown Origin (CPUO), a condition with significant unmet medical needs. The upcoming Phase II CPUO study highlights Galderma’s ongoing commitment to advancing treatment options for individuals affected by chronic skin conditions that substantially impact quality of life.
This randomized, double-blind, placebo-controlled proof-of-concept trial will assess the pharmacokinetics and pharmacodynamics of nemolizumab in adult participants. Enrollment is planned to begin in the U.S. during the second half of 2025, with study completion expected in 2026.
The trial was developed in collaboration with a Steering Committee of leading dermatology experts, including Dr. Shawn Kwatra, M.D., Ph.D., Joseph W. Burnett Endowed Professor and Chairman of Dermatology at the University of Maryland School of Medicine, and Dr. Sarina Elmariah, MD, Ph.D., MPH, Associate Professor and Director of the Center for Itch and Neurosensory Disorders at the University of California, San Francisco.
Mirum Pharmaceuticals’ Volixibat
Volixibat is an oral ileal bile acid transporter (IBAT) inhibitor with minimal systemic absorption, currently being studied for adult cholestatic conditions. It works by preventing bile acid reabsorption, aiming to reduce both systemic and liver bile acid levels, which may help mitigate disease-related complications. The US FDA has granted volixibat both Orphan Drug Designation (ODD) and Breakthrough Therapy Designation (BTD) for cholestatic pruritus in primary biliary cholangitis (PBC).
The drug is in Phase IIb trials targeting cholestatic pruritus, including the VISTAS study in primary sclerosing cholangitis and the VANTAGE study in PBC. Interim data were released in June 2024. VISTAS is expected to complete enrollment by Q3 2025, with topline results anticipated in Q2 2026, while VANTAGE enrollment is projected to conclude in 2026.
The introduction of these emerging chronic pruritus therapies is poised to reshape the chronic pruritus treatment landscape significantly. With novel mechanisms of action, such as IL-31 receptor antagonism and IBAT inhibition, patients are likely to experience more targeted and effective relief than currently available options. As these chronic pruritus drugs gain regulatory approvals and enter clinical practice, clinicians will have a broader arsenal to personalize therapy based on the underlying etiology of pruritus, disease severity, and comorbidities. This shift may also encourage earlier intervention, improved symptom management, and a reduction in the reliance on off-label or less-specific treatments, ultimately enhancing quality of life for patients struggling with chronic itch.
Moreover, the evolving pipeline is likely to stimulate competition and innovation within the market, accelerating the development of next-generation therapies and combination regimens. Real-world evidence generated post-launch will help identify optimal patient subgroups, refine dosing strategies, and expand indications beyond the initial approvals. Over time, these advancements could lead to a more stratified treatment approach, where immunologic, cholestatic, and neuroimmune pruritus types are addressed with distinct, mechanism-based therapies. As a result, the chronic pruritus landscape is set to transition from a largely symptom-focused paradigm to a precision-driven model, offering hope to patients who have long faced limited options.

Downloads
Article in PDF
Recent Articles
- ATS 2023 Updates: Dupixent – A Ray of Hope For Moderate To Severe Chronic Obstructive Pulmo...
- DUPIXENT Receives First-Ever Biologic Approval for COPD: Adds Another Jewel in its Crown
- Can Dupixent Be A Gamechanger In The Atopic Dermatitis Treatment Landscape?
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
- The Evolving Landscape of COPD Treatments: New Hope for Patients