A Glimpse of the Anticoagulants Therapy Market; Major Players
Apr 21, 2015
VTE therapeutics & Atrial Fibrillation market was worth USD 4 billion in 2014 and USD 7.86 billion respectively. The VTE therapeutic market is expected to reach USD 5.5 billion by 2018 with a Compound Annual Growth Rate (CAGR) of 8.29% and Atrial Fibrillation market will reach on its peak value USD 13.28 billion by 2018 with compound annual growth rate (CAGR) of 14%, according to DelveInsight Analysis.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Biosimilar Market in India
- AngioDynamics’s AlphaVac F18 PE System; Pfizer’s RSVpreF/PF-06928316 Trial; Intelligent Ultrasoun...
- Key Updates on Phase 1 Trial of AB-1005 Gene Therapy for Multiple System Atrophy-Parkinsonian Typ...
- CE Mark to Ibex’s Gastric Cancer Detection System; Senseonics’s Eversense E3 Continuous Glucose M...
- Alma Announced the Launch of Alma Duo; Phillips-Medisize Launched a Pen Injector Platform; FDA Ap...
Anticoagulants reduce blood clotting in an artery, a vein or the heart. Clots can block the blood flow to heart muscle leading to heart attack. They can also block blood flow to brain, leading to stroke. Anticoagulants are given to prevent blood from clotting or prevent existing clots from getting larger.
Warfarin and Heparin were the only available anticoagulants since decades but now, the choice of anticoagulants is broadened by the approval of new oral anticoagulants bu U.S. Food and Drug Administration. The new oral anticoagulants include Dabigatran, Rivaroxaban, Edoxaban, and Apixaban. These novel oral anticoagulants are expected to replace older anticoagulants with their ease of use and favourable pharmacodynamic profiles.
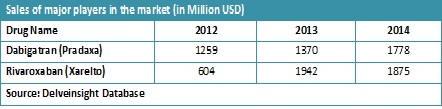
Dabigatran and Rivaroxaban were the highest selling drugs in 2014 with the revenue generated ~1800 million and ~1700 million respectively. Dabigatran was the first oral direct thrombin inhibitor approved by U.S. FDA and Rivaroxaban was the first oral direct coagulation factor Xa inhibitor approved by U.S. FDA. The patent for Dabigatran is going to expire soon (in 2017) thus making way for generic drug in the market. Edoxaban is the newly approved (January 2015) factor Xa inhibitor for the prevention of ischemic stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF) and the treatment and recurrence prevention of venous thromboembolism (VTE).
Despite novel anticoagulants are seen as replacements for heparin and warfarin, there are some adverse hemorrhagic events reported. Absence of any approved antidotes for oral anticoagulants limit their use. Though there are 3 antidotes in clinical development which can reverse the effects in case of major bleeding. These antidotes are 1.5 years away from being clinically available.
Since the patient compliance increases with the orally available drugs, the companies are focusing on developing such anticoagulants. The drugs in pipeline are mostly thrombin inhibitors or Factor Xa inhibitors unlike traditionally used warfarin which was Vitamin K reductase inhibitor.
Anticoagulants are prescribed for number of diseases such as Heart attack, stroke, deep venous thromboembolism, pulmonary embolism, Atrial fibrillation, Unstable angina, or for the prevention of Venous thromboembolism (VTE) after major orthopedic surgery. With such a wide usage, one must be familiar with anticoagulants, their pharmacological properties, pharmacodynamics, dosing, monitoring and toxicity.
Written by Rashi Aggarwal, Associate Analyst at DelveInsight
Report: Anticoagulants-Competitive Landscape, Market & Pipeline Analysis, Forecasted Market Size 2015-2018
Give your views in the comment section and for more information on this Report and Sample Pages mail us at info@delveinsight.com
Downloads
Article in PDF
Recent Articles
- CE Mark to Ibex’s Gastric Cancer Detection System; Senseonics’s Eversense E3 Continuous Glucose M...
- Alma Announced the Launch of Alma Duo; Phillips-Medisize Launched a Pen Injector Platform; FDA Ap...
- The Question That Remains Unanswered: What Might Be Causing Alzheimer’s?
- Valeant’s $50M; Sanofi vows; Top pharmas team up; PhRMA expels
- Integra to Buy J&J’s Acclarent; B. Braun Launches the CARESITE Micro Luer Access Devic; FDA ...