The AI Drug Discovery Revolution: Smarter Science, Faster Therapies, Better Outcomes
Oct 22, 2025
Table of Contents
The pharmaceutical industry has always been a high-stakes arena, where years of research, billions of dollars, and countless clinical trials culminate in a single therapy reaching patients. Despite tremendous advancements, the traditional drug discovery process remains time-consuming, costly, and fraught with uncertainty. Enter Artificial Intelligence (AI), a technological force poised to reshape every stage of drug development. From target identification to clinical trials and pharmacovigilance, AI is accelerating timelines, optimizing resources, and enabling smarter decision-making.
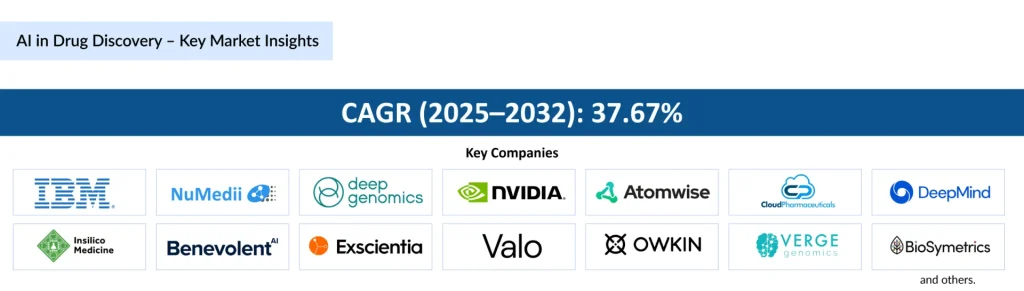
The AI in the drug discovery market reflects this transformative potential. Estimated to grow at a CAGR of 37.67% between 2025 and 2032, the market is projected to expand significantly as pharmaceutical companies, biotechnology startups, and technology giants embrace AI-driven approaches. By harnessing the computational power of machine learning, generative AI, and knowledge graphs, drug discovery is moving from a predominantly empirical process to a predictive, data-driven science.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- VARON’s New VP Series Portable Oxygen Concentrator; Biostrap’s Wrist-Worn Digital Health Monitori...
- From Science Fiction to Reality: Investigating the Market Dynamics and Growing Demand for the Sma...
- FDA declines the approval of Lipocine’s testosterone drug; Novartis Sandoz to buy Aspen Pharmaca...
- Who is Going to be a Trendsetter in the Hereditary Deafness Market?
- Hepion aces mid-stage study; Bicycle Therapeutics-Ionis collaboration; Nimbus raises $105M; Regen...
We have a separate blog on how AI is transforming diagnostics across healthcare. Read here: AI-Driven Diagnostics
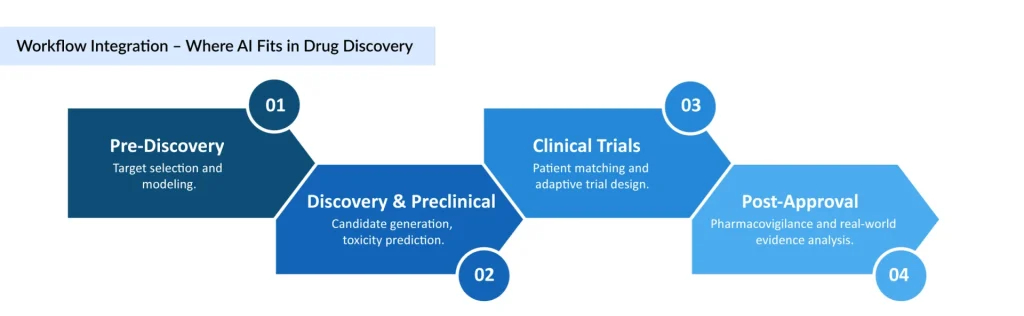
Why AI is a Game-Changer in Drug Discovery
The rationale for integrating AI into drug discovery is compelling. Chronic and complex diseases, ranging from cancer and cardiovascular disorders to neurological conditions, demand therapies that are effective, safe, and personalized. Traditional drug discovery, relying on trial-and-error experimentation and lengthy clinical validation, often struggles to keep pace with rising global healthcare needs. AI addresses these challenges by:
- Accelerating target identification: Machine learning models can analyze vast genomic and proteomic datasets to identify novel drug targets in a fraction of the time required by conventional methods.
- Optimizing molecular design: Generative AI algorithms create candidate molecules with higher predicted efficacy, better binding affinity, and fewer potential side effects.
- Enhancing predictive safety: AI-driven pharmacovigilance platforms detect adverse events early, reducing risks and improving patient safety.
- Streamlining clinical trials: By predicting patient responses and optimizing trial design, AI shortens timelines and enhances the probability of success.
These benefits are not hypothetical, they are being realized today through both established pharmaceutical giants and AI-focused startups.

Market Landscape and Regional Insights
Among all regions, North America dominates the AI in drug discovery market, thanks to a combination of factors. The region hosts a large patient pool across diverse disease areas, including oncology and neurology, driving demand for novel therapeutics. Moreover, the presence of leading pharmaceutical and technology companies, strong research infrastructure, and supportive regulatory frameworks has positioned North America as a global hub for AI-driven drug development.
Other regions, including Europe, Asia-Pacific, and emerging markets, are increasingly adopting AI technologies. Collaborations between local startups and multinational pharmaceutical companies are enhancing research capabilities, particularly in areas like computational chemistry, AI-guided clinical trials, and precision medicine.
Key market players contributing to this AI revolution include IBM Corporation, Numedii Inc., Deep Genomics, NVIDIA Corporation, Atomwise Inc., Cloud Pharmaceuticals Inc., Alphabet Inc. (DeepMind), Insilico Medicine, BenevolentAI, Exscientia, Cyclia, Valo Health, Owkin Inc., Verge Genomics, and BioSymetrics, among others. Their solutions range from AI-driven molecular design and predictive modeling to automated labs and knowledge graph integrations, collectively reshaping the discovery and development pipeline.
AI in Action: Breakthroughs and Recent Developments
Recent years have seen AI-powered drug candidates progress from concept to clinical trials at unprecedented speed:
- iRhythm Technologies Zio® ECG (September 2024): Received Japanese regulatory approval as the first AI-enabled arrhythmia monitoring system.
- Royal Philips + smartQare Partnership (April 2024): Integration of viQtor with Philips’ monitoring platforms to enable continuous patient monitoring in hospitals and remotely across Europe.
- Dozee (India, December 2024): Deployed contactless AI-RPM in hospitals for real-time monitoring, improving early deterioration detection.
- AI Early-Warning RPM (Kolkata, October 2024): Introduced scalable, privacy-conscious AI monitoring in urban hospitals, highlighting the growing innovation from India.
- INS018_055 and Rentosertib (February & March 2025): Developed by Insilico Medicine, these AI-designed drugs advanced to Phase 2 clinical trials in record timelines, Rentosertib achieved USAN status, moving from target discovery to Phase 2 in under 30 months.
- 5G + AI Hybrid Systems (January 2025): Demonstrated 14.4 ms latency and 96.5% accuracy in vital predictions, showcasing near real-time monitoring capabilities.
- Real-Time Anomaly Detection Models (February 2025): Deployed secure AI frameworks to identify anomalies in continuous clinical monitoring.
- Eko Health’s AI Stethoscope (August 2025): Detects cardiac abnormalities in seconds, validating AI’s diagnostic potential.
- Cleveland Clinic + Piramidal (August 2025): Launched an AI EEG system for continuous ICU monitoring, transforming neurocritical care with predictive seizure alerts.
Additionally, AI-driven platforms and collaborations are rapidly expanding:
- Nabla Bio and Takeda Pharmaceuticals (October 2025): Enhanced AI-driven drug design for protein-based therapeutics, accelerating Takeda’s early-stage pipeline.
- Lila Sciences’ AI Science Factories (October 2025): Raised $115 million to develop robotic labs guided by AI, creating an autonomous experimental ecosystem.
- K-DREAM (October 2025): Integrates biomedical knowledge graphs with generative AI to improve molecular generation and therapeutic targeting.
- IDOLpro (May 2024): Employs generative chemistry AI for structure-based drug design, optimizing binding affinity and synthetic accessibility.
- Graph AI (October 2025): Secured funding to enhance pharmacovigilance using AI, improving early detection of adverse drug reactions.
- NetraMark’s NetraAI 2.0 (February 2025): An advanced AI platform streamlining clinical trial analytics, demonstrating AI’s growing adoption in regulatory science.
Transforming Clinical Research: Efficiency Meets Precision
The integration of AI into drug discovery is transforming how clinical research is conducted. Traditionally, the attrition rate of drug candidates through preclinical and clinical stages has been high. By employing predictive modeling, AI identifies high-potential candidates early, reducing wasted resources and costs. Simultaneously, AI-assisted trial designs enable smarter patient selection, real-time monitoring, and faster endpoint assessment, thereby shortening trial durations without compromising scientific rigor.
For example, AI-powered analysis of electronic health records (EHRs) allows for predictive patient matching, ensuring trials enroll participants most likely to benefit from investigational therapies. Similarly, natural language processing (NLP) algorithms extract insights from unstructured clinical data, supporting evidence-based decision-making at every stage of development.
Explore how AI is improving patient care in real-time settings: Read here: The Rise of AI-Powered Point-of-Care Diagnostics
Challenges and Considerations
While AI promises transformative benefits, several challenges remain:
- Data Availability and Quality: High-quality, well-annotated datasets are critical for training AI models. Gaps or biases in data can limit predictive accuracy.
- Regulatory Compliance: AI-driven drug discovery tools must meet stringent FDA, EMA, and other regulatory requirements, particularly for clinical validation and patient safety.
- Integration with Existing Workflows: Incorporating AI into legacy R&D processes requires careful planning, personnel training, and change management.
- Intellectual Property and Collaboration: As AI designs novel compounds, questions arise regarding ownership, patentability, and licensing.
Addressing these challenges will require close collaboration between technologists, regulatory bodies, and life sciences experts, ensuring AI tools are safe, robust, and scalable.
The Future of AI in Drug Discovery
The next decade is poised to revolutionize drug discovery, with AI emerging as a key R&D engine. Generative design at scale will enable the creation of millions of molecular candidates, targeting multi-factorial diseases while optimizing efficacy, safety, and manufacturability.
Integration with digital twins, virtual patient models built from multimodal data, will allow researchers to simulate treatment responses, anticipate adverse effects, and personalize interventions before clinical trials. AI-driven platforms will continuously update themselves with the latest biomedical knowledge, ensuring that research remains at the forefront of innovation.
Cloud-based ecosystems and global collaboration platforms will democratize access to AI drug discovery tools, enabling smaller biotech firms and academic institutions to compete on innovation while fostering multi-institutional partnerships. Additionally, AI will increasingly support value-based R&D strategies, helping companies focus on high-need therapeutic areas, allocate resources efficiently, and improve patient outcomes.
Ethical frameworks and regulatory guidance will evolve alongside AI, ensuring safety, transparency, and fairness. Explainable AI (XAI) will provide interpretability for clinicians and regulators, while robust privacy and data governance measures will safeguard patient information.
Ultimately, AI will transform drug discovery from a reactive, trial-and-error process to a predictive, patient-centric, and precision-driven endeavor, delivering therapies faster, reducing development costs, and addressing unmet medical needs at scale.
Conclusion
Artificial Intelligence is no longer a futuristic concept in drug discovery, it is an operational reality reshaping how science is conducted. From AI-designed molecules reaching clinical trials in record time to predictive platforms optimizing patient selection and trial outcomes, the industry is experiencing a paradigm shift. With continued investments, technological refinement, and responsible deployment, AI has the potential to make drug development more innovative, more efficient, and more patient-centric. For pharma stakeholders, CXOs, and healthcare innovators, embracing AI is no longer optional; it is a strategic imperative for the next era of therapeutic innovation.

Downloads
Article in PDF
Recent Articles
- Assessing the Emerging Trends in the Evolving Implantable Drug Delivery Device Landscape
- Unlocking New Avenues in KRAS-Driven Cancer Research Beyond G12C
- The Business Cocktail
- How the Amniotic Membrane Market is Transforming Regenerative Healthcare Worldwide
- Relievant Medsystems Launched Ablation System; Thermo Fisher’s Reproductive Health Assays; EarliT...