The Rise of AI-Powered Point-of-Care Diagnostics: Transforming Real-Time Patient Care
Sep 17, 2025
Table of Contents
Point-of-care (POC) diagnostics have revolutionized healthcare by enabling rapid, on-site testing that delivers actionable results without the delays of traditional laboratory processes. For instance, over 1.5 billion POC tests are performed annually worldwide, ranging from portable blood glucose monitors to point-of-care molecular diagnostics. These tools empower clinicians to make informed decisions at the patient’s side, whether in hospitals, clinics, or remote settings. The point-of-care diagnostics market has seen substantial growth, driven by the need for accessible, timely, and cost-effective testing solutions. As healthcare systems worldwide prioritize efficiency and patient-centered care, POC diagnostics have become a cornerstone of modern medical practice, bridging the gap between diagnosis and treatment.
The integration of artificial intelligence into point-of-care diagnostics marks a transformative leap forward, enhancing the precision and utility of these technologies. AI-powered systems leverage advanced algorithms and machine learning to analyze complex datasets, improving diagnostic accuracy and enabling real-time decision-making. The point-of-care molecular diagnostics market, in particular, has benefited from AI’s ability to interpret intricate molecular data swiftly, facilitating faster identification of conditions such as infectious diseases or genetic disorders.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Assessing the Major Growth and Ongoing Developments in the Clinical Diagnostics Market
- Ascendis’s SKYTROFA; MicroTransponder’s VNS System (Vivistim); BioMarin’s Voxzogo; Zi...
- Future Avenues for Prediabetes Treatment: The Road Ahead
- HCQ/ CQ failure; AZ/ Catalent’s deal; Sinovac’s CoronaVac and Lilly’s Olumiant trial results
- CEPI Grants $41.3 Million to Valneva; Innovent Achieves Phase III Success for Mazdutide; GSK’s BL...
By combining the portability of POC devices with the analytical power of AI, healthcare providers can deliver more personalized and effective care, setting the stage for a new era in point-of-care diagnostic innovation.
Common Applications of AI in Point-of-Care Diagnostics
| Application Area | Example Use Case | AI Contribution |
| Infectious Diseases | Rapid COVID-19 testing | AI interprets lateral flow assays |
| Radiology | Handheld ultrasound devices | AI-assisted image analysis for pneumonia, fractures |
| Cardiology | Wearable ECG monitors | AI detects arrhythmias like atrial fibrillation |
| Ophthalmology | Retinal imaging | AI identifies diabetic retinopathy on portable cameras |
| Primary Care | Symptom checker apps | AI triages based on inputs & suggests diagnostics |
AI Integration in Point-of-Care Diagnostics
Artificial intelligence (AI) and machine learning (ML) are revolutionizing point-of-care (POC) diagnostics by significantly enhancing diagnostic precision and reliability. These technologies process complex datasets from POC devices, including biosensors, imaging systems, and molecular assays, to detect subtle patterns and anomalies that traditional methods might overlook. For example, machine learning models can reduce diagnostic errors in point-of-care molecular diagnostics by up to 20%, according to recent studies, by refining biomarker analysis and minimizing false positives and negatives.
By enabling real-time, data-driven insights, AI empowers clinicians to make accurate, evidence-based decisions at the patient’s side, particularly in critical scenarios like infectious disease detection or acute condition management, where speed and precision are paramount.
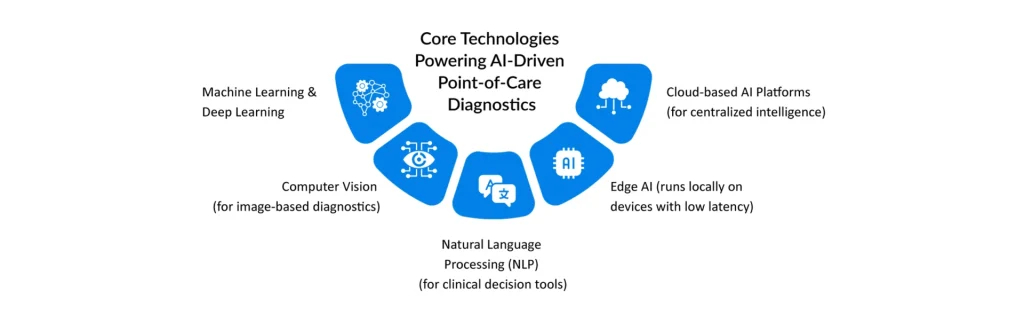
Examples of AI-Powered POC Tools
AI-powered POC tools are reshaping healthcare delivery with innovative solutions that combine portability and advanced analytics. Key examples include:
- AI-Enhanced Portable Ultrasound Devices: These devices use image recognition algorithms to guide non-specialist clinicians in interpreting scans, achieving diagnostic accuracy comparable to expert radiologists, even in remote settings.
- ID NOW Platform by Abbott: This POC molecular diagnostics tool leverages AI to deliver rapid and precise results for diseases like COVID-19 and influenza, providing actionable outcomes in under 15 minutes.
- Smartphone-Based Diagnostic Apps: Apps utilizing AI-driven image analysis enable users to diagnose conditions such as skin lesions or blood abnormalities by processing high-resolution images, expanding access to diagnostics in low-resource environments.
- Wearable Biosensors with AI Integration: Devices like continuous glucose monitors use AI to predict trends and provide real-time alerts, improving chronic disease management at the point of care.
These examples highlight how AI is elevating the capabilities of POC diagnostics, making them more accessible, accurate, and impactful across diverse healthcare settings.
Point-of-Care Diagnostics Recent Developments
The field of AI-powered point-of-care (POC) diagnostics has witnessed remarkable progress in recent years, with biotech companies driving innovation through regulatory approvals, technological breakthroughs, and novel applications. These advancements are enhancing the capabilities of POC diagnostics, particularly in point-of-care molecular diagnostics, by leveraging artificial intelligence to deliver faster, more accurate, and accessible testing solutions. Below, we highlight key developments from leading biotech companies, showcasing how AI is reshaping the POC diagnostics landscape.
October 2024
NOWDiagnostics & Labcorp Collaboration
NOWDiagnostics, Inc. announced a strategic partnership with Labcorp to distribute its First To Know® Syphilis Test across professional healthcare settings in the U.S. The rapid test delivers results in 15 minutes using a drop of blood. Labcorp will offer the test in clinical settings by the end of 2024, with broader access via Labcorp OnDemand in 2025.
January 2025
Roche – cobas® pulse System Launch
Roche launched the cobas® pulse, an AI-powered point-of-care diagnostic platform designed for real-time blood biomarker analysis, including glucose monitoring. Cleared by the FDA in late 2024, the system offers decision-support tools for managing chronic diseases like diabetes.
Roche – STI Multiplex Assay Clearance
Roche received FDA 510(k) clearance and a CLIA waiver for its cobas liat STI multiplex assay panels, enabling rapid diagnosis of STIs such as chlamydia, gonorrhea, and Mycoplasma genitalium. Results are delivered in 20 minutes, streamlining diagnosis and treatment in decentralized settings.
CovarsaDx & Nuclein – DASH® SARS-CoV-2 & Flu A/B Test
CovarsaDx announced FDA 510(k) clearance and CLIA waiver for Nuclein’s DASH® SARS-CoV-2 & Flu A/B Test, designed for use on the DASH® Rapid PCR System. This test enables rapid, on-site detection of COVID-19 and influenza at the point of care.
February 2025
Thermo Fisher Scientific – AI-Powered Portable Analyzer
Thermo Fisher introduced an FDA-cleared AI-integrated portable analyzer for point-of-care molecular diagnostics in infectious diseases. This system delivers results in just 15 minutes, supporting rapid outbreak response in remote areas.
Aptitude Medical Systems – Metrix® COVID/Flu Test EUA
Aptitude Medical received Emergency Use Authorization (EUA) from the FDA for its Metrix® COVID/Flu multiplex test. This over-the-counter molecular diagnostic test detects and differentiates between SARS-CoV-2, Influenza A, and B within 20 minutes, suitable for at-home or CLIA-waived POC settings.
April 2025
QIAGEN – QIAstat-Dx AI Enhancement
QIAGEN enhanced its QIAstat-Dx respiratory panel with AI algorithms, improving sensitivity by 25% for faster and more accurate identification of respiratory pathogens at the point of care.
Baebies – Breakthrough Device Designation for Anti-Factor Xa Test
Baebies received FDA Breakthrough Device Designation for its Anti-Factor Xa test on the FINDER® platform—the first POC heparin monitoring assay. Delivering results in under 15 minutes from just 50 µL of blood, the test supports faster dose adjustments in critical care.
June 2025
Cepheid – GeneXpert System AI Enhancements
Cepheid announced AI-based enhancements to its GeneXpert System, enabling improved multiplexing and faster, more accurate detection of respiratory pathogens. The updates reduce diagnostic time to under 20 minutes, supporting POC infectious disease testing.
July 2025
BD – FDA Clearance for BD Veritor™ COVID-19 Antigen Test
BD (Becton, Dickinson and Company) received FDA 510(k) clearance for its BD Veritor™ System for SARS-CoV-2, a digital antigen test delivering results in about 15 minutes. Designed for use in clinics, urgent care centers, and other POC settings, the test aids rapid COVID-19 detection in symptomatic individuals.
August 2025
Sonic Incytes – FDA Clearance for Velacur ONE™
Sonic Incytes Medical Corp received FDA 510(k) clearance for Velacur ONE™, a next-generation POC ultrasound elastography device for managing chronic liver diseases such as MASH and MASLD. The system features enhanced portability and a user interface, measuring liver stiffness, attenuation, and VDFF using advanced 3D S-WAVE technology.
These developments underscore the rapid evolution of AI-powered point-of-care diagnostics, with biotech companies leading the charge in delivering faster, more accurate, and accessible testing solutions.
From molecular assays to AI-enhanced imaging and wearable biosensors, the point-of-care diagnostics market is witnessing a surge in innovation that is redefining patient care.
As these technologies continue to advance, they promise to empower clinicians with real-time insights, streamline decision-making, and expand access to high-quality diagnostics across diverse healthcare settings.
Point-of-Care Diagnostics Market Growth Drivers and Emerging Trends
The point of care diagnostics market is on a dynamic growth trajectory, driven by the increasing demand for rapid, accessible, and accurate point of care diagnostic solutions. Valued for delivering results at or near the patient’s location, the global point of care diagnostics market is projected to grow at a compound annual growth rate (CAGR) of 6.61% from 2025 to 2032, propelled by the rising prevalence of chronic conditions like diabetes and cardiovascular diseases, as well as infectious diseases.
The point-of-care molecular diagnostics market has seen particular growth, especially post-COVID-19, as rapid testing solutions like antigen tests proved vital in managing public health crises. North America, with its advanced healthcare infrastructure and key players like F. Hoffmann-La Roche Ltd., Abbott, and Danaher, held the largest share of the point of care diagnostics market in 2024 and is expected to lead through 2032.

Key factors shaping the point of care diagnostics market include:
- Growth Projections: The point of care diagnostics market is forecasted to grow significantly with North America dominating due to its sophisticated healthcare systems and robust product development by companies like BD and Siemens Healthcare.
- Adoption Drivers: The rising prevalence of lifestyle-related diseases, such as diabetes requiring regular point of care diagnostic monitoring, and infectious diseases necessitating point of care molecular diagnostics, drives demand. Growing health awareness and the cost-effectiveness of non-invasive or minimally invasive diagnostics compared to lab-based tests further boost adoption.
- Emerging Trends: Integration of AI and machine learning is enhancing the accuracy of point of care diagnostic tools, while multiplexed platforms enable simultaneous pathogen detection. Telehealth-compatible point of care diagnostics, such as wearable biosensors, are gaining popularity for remote monitoring, especially for chronic disease management.
- Post-COVID Impact: The pandemic significantly increased demand for point of care diagnostic solutions, with rapid antigen testing kits like Abbott’s ID NOW highlighting the scalability and importance of the market in crisis response.
Despite challenges such as potential inaccuracies in diagnostic results, the point of care diagnostics market is set to thrive, driven by technological innovations and a shift toward patient-centric healthcare. Companies like Thermo Fisher Scientific, Quest Diagnostics, and Quidel Corporation are leading the charge, advancing point of care diagnostics to meet global healthcare needs.
Accelerating Patient Care with AI-Driven Point-of-Care Diagnostics
The integration of artificial intelligence into point of care diagnostics is transforming patient management by enabling rapid, accurate, and actionable results at the patient’s side. Real-time insights from point of care molecular diagnostics allow clinicians to make informed decisions instantly, whether for infectious disease detection, chronic condition management, or acute care scenarios.
Patients benefit from faster diagnoses, reduced hospital stays, and improved treatment outcomes. AI-powered POC devices also facilitate telehealth integration, enabling remote monitoring and virtual consultations, which is especially critical in rural or resource-limited settings. By bringing precision diagnostics directly to the point of care, healthcare systems can enhance patient-centered care while improving operational efficiency.
Navigating Challenges in the Point-of-Care Diagnostics Market
The growth of the point-of-care diagnostics market comes with unique challenges that need careful attention:
- Regulatory hurdles: FDA approvals, CLIA waivers, and stringent safety standards are essential to ensure device reliability and patient safety.
- Data privacy and security: AI-powered POC platforms generate sensitive patient data, requiring compliance with HIPAA, GDPR, and other data protection regulations.
- Accessibility and infrastructure: High costs and limited availability of advanced devices can hinder adoption in low-resource settings, slowing the impact of point of care molecular diagnostics.
Addressing these challenges is crucial to ensuring safe, accurate, and widely accessible point of care diagnostics that meet the growing demand for decentralized, patient-centric healthcare solutions.
Future Perspectives: Shaping the Next Era of Point-of-Care Diagnostics
The future of point of care diagnostics is poised for remarkable growth, driven by continuous innovation, AI integration, and a global shift toward patient-centered care. As devices become smarter, faster, and more accessible, healthcare providers will increasingly rely on AI-powered POC solutions to deliver timely, personalized, and actionable insights. This evolution promises to bridge the gap between diagnosis and treatment, empower clinicians with real-time decision-making tools, and ultimately improve outcomes for patients worldwide. The next era of point of care molecular diagnostics will not only redefine how care is delivered but also make precision medicine a tangible reality at every patient’s bedside.

FAQs
AI is making POC tools smarter by helping them analyze complex data in real time. Instead of just giving a basic reading, AI-powered devices can detect subtle patterns, reduce errors, and provide actionable insights instantly. This means faster, more reliable decisions right at the patient’s bedside, whether in a hospital, clinic, or even a rural health camp.
Not exactly, but they are becoming a strong complement. While labs still play a key role for in-depth testing, AI-driven POC devices bridge the gap by delivering rapid, on-the-spot results. For many conditions like diabetes, infectious diseases, or even heart rhythm disorders, clinicians don’t need to wait days for lab reports; they can act immediately.
Think of portable ultrasound machines that use AI to guide non-specialists, smartphone apps that scan skin lesions for early cancer signs, or wearable sensors that predict blood sugar spikes before they happen. Companies like Abbott (with ID NOW), Roche (cobas® pulse), and BD (Veritor™) are already bringing such innovations into daily healthcare.
Because speed and accessibility matter more than ever. The global POC diagnostics market is projected to grow at a 6.61% CAGR from 2025 to 2032, fueled by rising chronic conditions like diabetes and heart disease, plus the continued need for rapid infectious disease testing post-COVID. Major players driving this growth include F. Hoffmann-La Roche Ltd., BD, Danaher, Abbott, Siemens Healthcare, Thermo Fisher Scientific, and Quest Diagnostics.
Imagine a world where a handheld device can scan, analyze, and give personalized treatment suggestions in minutes, even in the most remote locations. With AI getting better at interpreting data, the future will see POC diagnostics not only speeding up detection but also making precision medicine more accessible, bridging the gap between diagnosis and treatment like never before.
Downloads
Article in PDF
Recent Articles
- Bristol Myers’ Opdivo combo Opdualag for Melanoma; Biogen’s Aduhelm; Marinus’ Ztalmy for CDKL-5 D...
- AbbVie & Genmab’s Collaboration; Lilly’s antisense neuro deal; COVID-19 treatment upda...
- NICE endorsement for Pfizer’s Vizimpro; Eisai, Dundee targets Cancer; Capital raise for AgaMatrix
- Evaluating the Upcoming Drugs in Pipeline for Major Autoimmune Diseases
- Ofev’s expanded use; Arkin Bio-Ventures II launch; USD 125 M for COVID-19 treatment



