All Blogs
Sep 20, 2022
AstraZeneca’s Danicopan Trial; CHMP Recommends Sanofi/AstraZeneca’s nirsevimab; Akero’s NASH Drug Trial; FDA Grants Orphan Drug Status to SY-5609; BMS’s Opdivo Trial Results; Pfizer to File for FDA Approval for Meningitis Vaccine; EMA Orphan Drug Designation to CAN-2409; FDA Starts Priority Review of Chiesi ‘s velmanase alfa
AstraZeneca’s Danicopan Shows Positive Results in Phase III Trial Danicopan, an oral Factor D inhibitor developed by AstraZeneca, was expected to fail a phase II trial in rare kidney disease in 2020, but a new readout could revive the drug. Danicopan (ALXN2040) has demonstrated efficacy as an adjunct treatment f...
Read More...
Sep 19, 2022
Adial’s Micro-formulation: Opening New Massive Alcohol Use Disorder Treatment Market Opportunities
Key Highlights Through the use of genetic screening, i.e., a companion genetic bio-marker intended to identify patients most likely to respond to therapy, AD04 humanizes the alcohol use disorder treatment, which could lead to an increase in the usage of AD04Without the need for abstinence, AD04’s novel mechanism...
Read More...
Sep 16, 2022
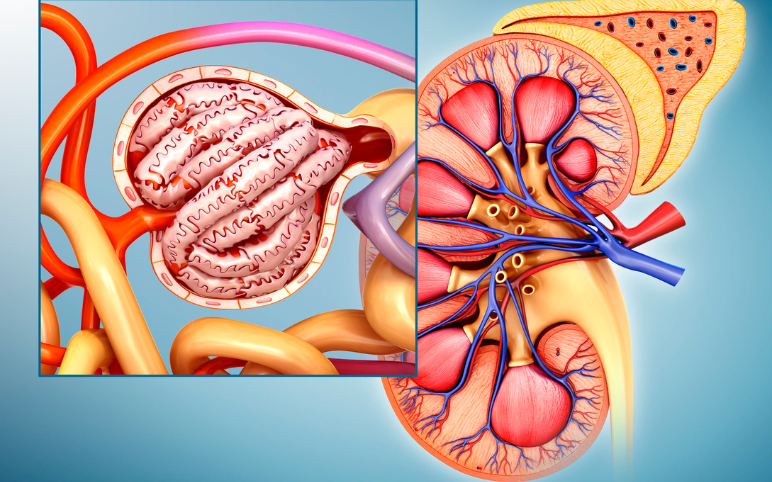
Focal Segmental Glomerulosclerosis Market: Unfolding Insights into the Future
Currently, there are no FDA-approved therapies for FSGS. However, the therapeutic focal segmental glomerulosclerosis market in the seven major geographies mainly comprises off-label focal segmental glomerulosclerosis treatment options. The off-label therapies majorly include nonimmune therapies in conjunction wi...
Read More...
Sep 15, 2022
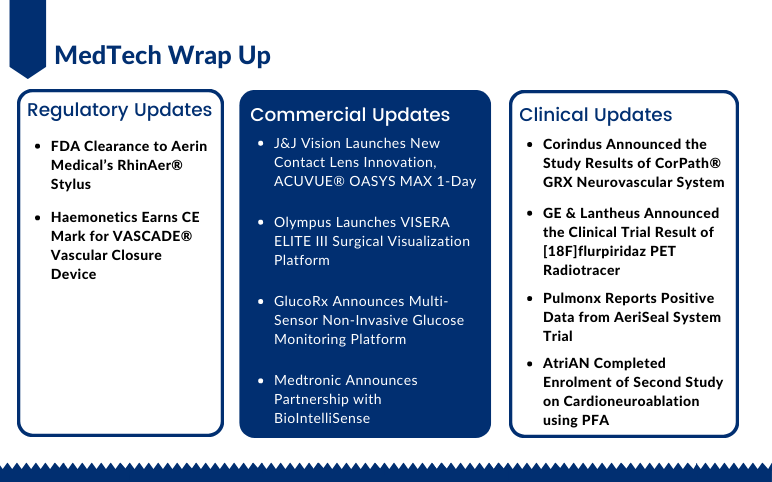
Aerin Medical’s RhinAer Stylus; CE Mark for Haemonetics’s VASCADE Vascular Closure Device; J&J Vision Launches ACUVUE OASYS MAX 1-Day; Olympus Launches VISERA ELITE III Surgical Visualization Platform; GlucoRx’s Non-Invasive Glucose Monitoring Platform; Medtronic-BioIntelliSense’s Partnership; Corindus’s CorPath GRX Neurovascular System Trial; GE & Lantheus [18F]flurpiridaz PET Radiotracer Trial; Pulmonx’s AeriSeal System Trial; AtriAN’s Second Study on Cardioneuroablation
Johnson & Johnson Vision Launches New Contact Lens Innovation ACUVUE® OASYS MAX 1-Day for Meeting the Needs of Digitally Intense Lifestyles On September 12, 2022, Johnson & Johnson Vision, a part of Johnson & Johnson and a global leader in the eyecare market, had announced the launch of its newest in...
Read More...
Aug 28, 2025
Navigating the Most Use Cases of Robotic Process Automation (RPA) in Healthcare
Robotic Process Automation (RPA) in healthcare involves utilizing software bots to automate routine and repetitive tasks, such as data entry, billing, scheduling, and claims processing. These bots mimic human actions on digital systems, aiming to enhance efficiency, minimize manual errors, and enable healthcare wor...
Read More...
Sep 13, 2022
Amgen Announced the Result of its CodeBreak-200 Trial; FDA Clears Bristol-Myers Squibb’s Deucravacitinib; BioNTech’s Amplified CAR-T Therapy; TIL Therapy Improves on Yervoy in Melanoma; GSK’s Daprodustat will have to face FDA Advisory Committee; Breakthrough Therapy Status to Pfizer’s Group B Strep Vaccine; EU Approves Gilead’ Tecartus; Gilead’ Trodelvy Results in TROPiCs-02 Trial
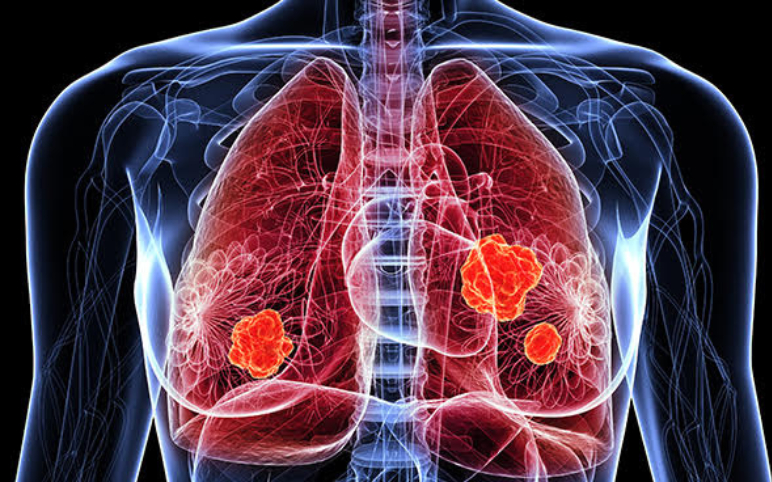
Amgen Reveals the Top-line Result of its CodeBreak-200 trial of Lumakras in Lung Cancer The top-line result of Amgen's CodeBreak-200 trial of Lumakras in lung cancer was presented in abstract form at ESMO two weeks ago, showing a 34% improvement in progression-free survival (PFS) compared to chemotherapy. The fu...
Read More...
Sep 12, 2022
How Emerging Pipeline Therapies Will Unfold the Severe Asthma Treatment Market Dynamics?
Key Highlights Tezspire (tezepelumab) with no phenotypic (eosinophilic or allergic) or biomarker restriction will be the new game-changer in the severe asthma marketDepemokimab is an excellent market replacement for GSKs’ Nucala as of its improved binding affinity and longer duration of action as a single doseNo...
Read More...
Sep 09, 2022
Treatment to Clinical Development – A Bumpy Journey for Pancreatic Cancer
Treatment for pancreatic cancer patients is challenging and not well defined, especially for patients with disease progression or adverse events seen after initial treatment. Studies suggest that, after diagnosis, about 22% of pancreatic cancer patients, regardless of their clinical stage of cancer, receive no trea...
Read More...
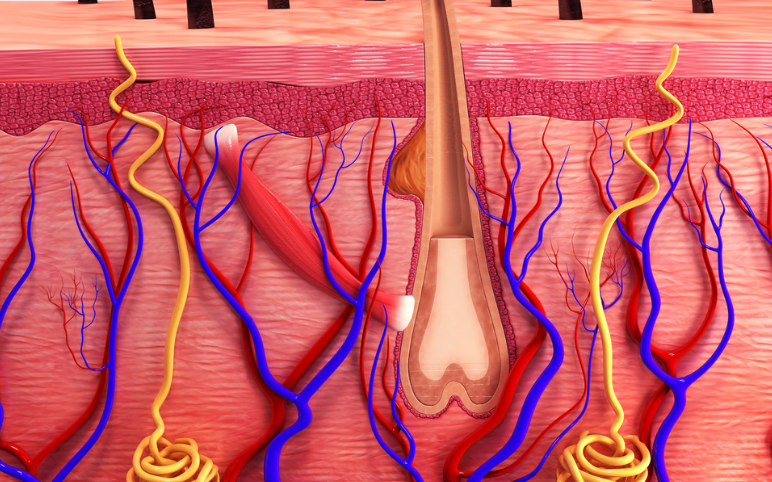
Sep 09, 2022
Alopecia Areata Treatment: Which Pipeline Therapy Will Steal the Spotlight?
Currently, there is no cure and no FDA-approved treatments for alopecia. Thus, the alopecia areata treatment market is completely dominated by off-label drug therapies. Depending on the extent of hair loss and age, there are a variety of alopecia areata treatment options are available. The main goal of treatment is...
Read More...
Sep 08, 2022
eCential Robotics’s Surgical Robotic Platform for Spine Surgery; Baxter Gets 510(k) Clearance for Syringe Pump; Medical Microinstruments’s NanoWrist Instruments; Magnus’ SAINT Neuromodulation System; Henry Schein Acquires Midway Dental Supply
eCential Robotics Receives FDA Clearance for its Surgical Robotic Platform for Spine Surgery The FDA has approved a robotic spinal surgery platform designed to assist human surgeons by automating several steps of spinal procedures. The platform, developed by eCential Robotics, combines intraoperative 2D and 3D i...
Read More...