All Blogs
Oct 04, 2022
Zealand Pharma’s Phase III Results of Glepaglutide; FDA Approves Amylyx’s ALS Drug Relyvrio; Novo Nordisk and Ventus Therapeutics Signs Licencing Deal; FDA Approves Futibatinib; Sarepta Files Duchenne Muscular Dystrophy for FDA Approval; Biogen and Eisai’s Lcanemab Phase III Study
Zealand Pharma Announces the Positive Topline Results from its Phase III trial of Glepaglutide Zealand Pharma A/S, a biotech company specializing in peptide-based medicines, announced positive topline results from its phase III trial of glepaglutide. In the evenly randomized double-blind trial, 106 patients with...
Read More...
Oct 03, 2022
Insights Into The Cutaneous T-cell Lymphoma Treatment Market
Cutaneous T-cell lymphomas (CTCLs) constitute a group of non-Hodgkin lymphomas of the skin. CTCL has an annual age-adjusted incidence of approximately 6 cases per million people. In the United States, approximately 1,000 new cases of skin lymphoma are diagnosed each year. CTCL affects males twice as females. Moreov...
Read More...
Oct 18, 2024
How are Antipsychotics Transforming the Schizophrenia Treatment Space?
Schizophrenia affects approximately 24 million people worldwide, or 1 in every 300 people (0.32%). In adults, this rate is 1 in 222 (0.45%). It is not as widespread as many other mental disorders. Onset occurs most frequently in late adolescence and the twenties, and it occurs earlier in men than in women. Schiz...
Read More...
Sep 29, 2022
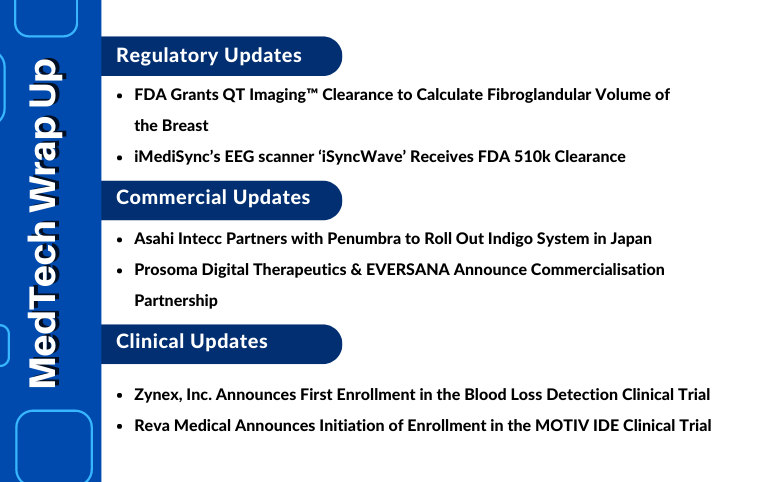
Asahi Intecc Partners with Penumbra; Prosoma and EVERSANA Announce Commercialisation Partnership; Zynex Starts Enrollment in the Blood Loss Detection Clinical Trial; Reva Initiates Enrollment in the MOTIV IDE Clinical Trial; FDA Clearance to QT Imaging to Calculate Fibroglandular Volume of the Breast; FDA 510k Clearance to iMediSync’s EEG scanner ‘iSyncWave’
Asahi Intecc partners with Penumbra to roll out Indigo System in Japan On September 22, 2022, Asahi Intecc, a Surgical and medical instrument manufacturing company, partnered with Penumbra, a medical device company headquartered in Alameda, California, to roll out Indigo System in Japan. After getting regulat...
Read More...
Sep 28, 2022
Evaluating the Growing Role of Devices in the Rehabilitation of Hearing Impairment
Hearing loss is a growing public health concern, and the number of cases is rising globally at a significant pace. As per the WHO, more than 1.5 billion people (nearly 20% of the global population) live with hearing loss, and 430 million of them have disabling hearing loss. According to the NIDCD, in the United Sta...
Read More...
Sep 27, 2022
Daiichi Sankyo’s Ezharmia; Pfizer & Sangamo Hemophilia A Gene Therapy Trial; Approval for Fennec’s Pedmark; FDA Approves UBE and Santen’s OMLONTI; EC Approves AstraZeneca’s Tezspire; FDA Approves Selpercatinib; FDA Grants Accelerated Approval to Eli Lilly’s Retevmo; GSK & Spero Announce Exclusive License Agreement
Daiichi Sankyo Receives the First Approval for its Blood Cancer Drug Ezharmia Daiichi Sankyo has received the first global approval for Ezharmia, a first-in-class dual EZH1 and EZH2 inhibitor for the treatment of patients with relapsed or refractory adult T-cell leukemia/lymphoma (ATL). The Japanese Ministry of ...
Read More...
Sep 26, 2022
PDE4-B Inhibitors: A Promising Target for Idiopathic Pulmonary Fibrosis Treatment
Idiopathic pulmonary fibrosis (IPF) is a rare, sporadic, and fatal interstitial lung disease. As the morbidity and mortality rates associated with IPF remain high, prompt idiopathic pulmonary fibrosis treatment is critical to safeguard individuals’ lung function, reduce the risk of acute exacerbations, and improve ...
Read More...
Jul 14, 2025
How Novel Therapies Could Transform the Ulcerative Colitis Treatment Landscape
Almost two decades ago, there were limited options for ulcerative colitis treatment. Choosing the right ulcerative colitis drugs depends on the severity of the condition and the patient's response to previous therapies. To manage patients effectively, physicians often prescribe a combination of ulcerative colitis m...
Read More...
Sep 22, 2022
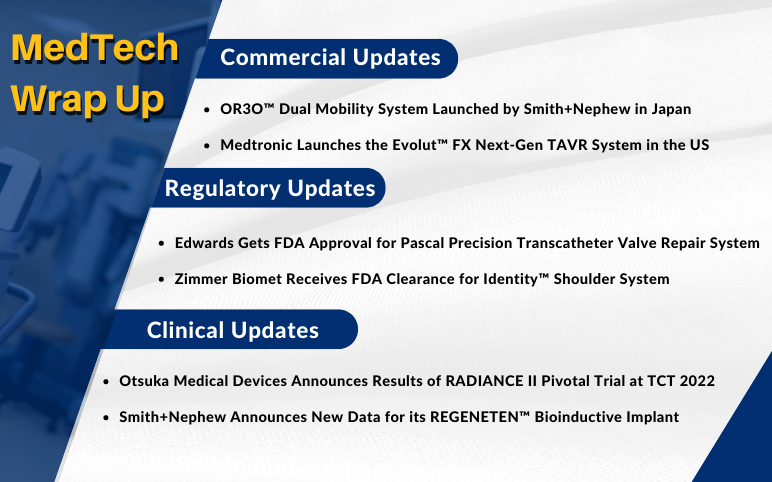
Smith+Nephew’s OR3O Dual Mobility System; Medtronic Launches the Evolut FX Next-Gen TAVR System; FDA Approves Edwards’s Pascal Precision Transcatheter Valve Repair System; FDA Clearance to Zimmer Biomet’s Identity Shoulder System; Otsuka Announces Results of RADIANCE II Trial; Smith+Nephew Announces Data for its REGENETEN™ Bioinductive Implant
OR3O™ Dual Mobility System launched by Smith+Nephew in Japan for use in primary and revision hip arthroplasty On September 20, 2022, Smith+Nephew, a leading global portfolio medical technology business, announced the launch of OR3O Dual Mobility System for use in primary and revision hip arthro...
Read More...
Oct 06, 2025
Brain-Computer Interface (BCI) Applications Driving Healthcare Innovation
Brain-Computer Interfaces (BCIs) are emerging as a groundbreaking technology in the healthcare sector, offering innovative solutions for patients with neurological disorders, spinal cord injuries, and other debilitating conditions. A BCI is a direct communication pathway between the brain and external devices, enab...
Read More...