Dry Eye Disease Treatment: A Growing Space with Expanding Therapeutic Options
Jun 02, 2025
Table of Contents
Dry eye disease (DED) is a common yet often underestimated condition affecting millions worldwide. Characterized by insufficient tear production or poor tear quality, it leads to persistent eye discomfort, irritation, and vision problems. Studies suggest that up to 30% of the adult population may experience some degree of dry eye symptoms, with prevalence increasing with age and certain environmental factors.
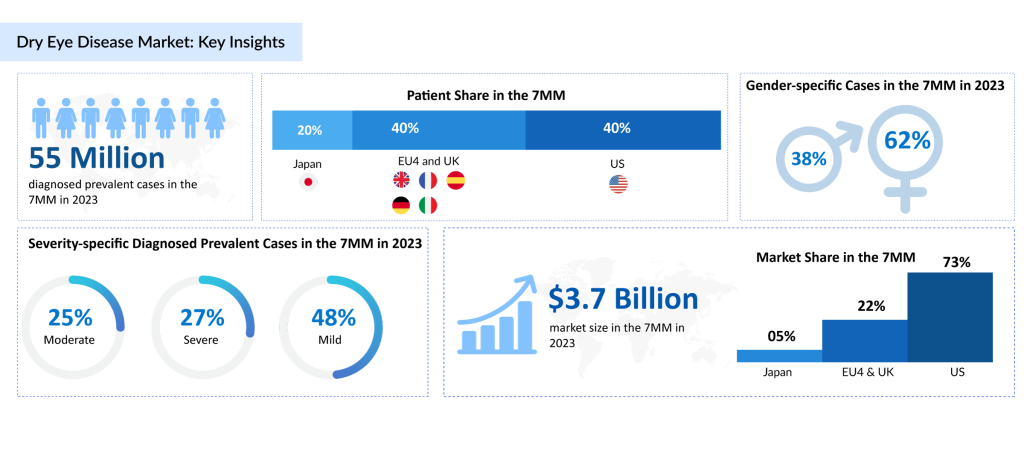
According to DelveInsight’s analysis, the diagnosed prevalent cases of DED in the 7MM were ~55 million in 2023. These cases are expected to increase at a significant CAGR throughout the study period (2020–2034). Mild cases of DED may be more common, as individuals with mild symptoms may not always seek medical attention. In contrast, moderate and severe cases, though less prevalent, can significantly impact quality of life. DelveInsight’s estimates project an increase in severity-specific DED cases over the coming years.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to S...
- Upcoming Contenders in Dry Eye Disease Drug Space: 5 Therapies to Watch Post-TRYPTYR Approval
- Alcon Gains CE Mark for Clareon Vivity IOL in Europe; MicroPort MedBot’s Toumai SP Robot Wins NMP...
- Cerner, Scanwell Health and Personal Genome Diagnostics Acquisition; Zynex’s Fluid Monitori...
- Leo Pharma’s Hand Eczema Clinical Trial Updates; GSK’s PD-1 inhibitor Jemperli Approval; Ro...
Treating dry eye syndrome requires a combination of methods: using over-the-counter artificial tears, prescription drugs such as RESTASIS or XIIDRA, procedures like LipiFlow, lifestyle adjustments including using humidifiers and taking omega-3 supplements, and, for more severe cases, options like punctal plugs or scleral lenses. Identifying the condition early and starting treatment promptly can help avoid complications such as corneal ulcers and scarring.
Currently Available Therapies for Dry Eye Disease Treatment
Various drugs have gained approval in the United States, Europe, and Japan for treating and managing DED. These dry eye disease drugs include CEQUA (Sun Pharmaceutical), TYRVAYA (OYSTER POINT PHARMA), EYSUVIS (Alcon Labs/Kala Pharmaceuticals), VEVYE (Novaliq/Harrow Eye), and NOV03 (Bausch & Lomb Incorporated/Novaliq).
CEQUA (cyclosporine ophthalmic solution) 0.09%, developed by Sun Pharmaceutical, is a sterile, clear solution designed to boost tear production in patients with keratoconjunctivitis sicca. Taken twice daily, it functions as a calcineurin inhibitor and immunosuppressant, potentially reducing ocular inflammation and partially modulating the immune response to alleviate dry eye symptoms.
OYSTER POINT PHARMA offers TYRVAYA (varenicline solution) nasal spray, a prescription treatment for dry eye disease. Administered twice daily, it works by stimulating nicotinic acetylcholine receptors, activating the trigeminal parasympathetic pathway to increase basal tear production, thereby relieving dry eye discomfort. Viatris has finalized its acquisition of Oyster Point Pharma.
EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25%, developed by Alcon Labs/Kala Pharmaceuticals, is FDA-approved for short-term relief of dry eye disease symptoms. Employing Kala’s AMPPLIFY Mucus-Penetrating Particle technology, it enhances penetration of loteprednol etabonate into eye tissues, providing rapid symptom relief with a strong safety profile.
VEVYE, from Novaliq/Harrow Eye, is an innovative dry eye treatment featuring cyclosporine solubilized in a water-free formulation. Approved by the FDA in June 2023, it delivers fast-acting relief without water, antimicrobial preservatives, oils, or surfactants. VEVYE is the first cyclosporine-based therapy targeting both signs and symptoms of dry eye disease, dosed as one drop twice daily per eye.
NOV03, developed by Bausch & Lomb/Novaliq and FDA-approved in 2023, is a water-free, preservative-free eye drop for dry eye disease linked to Meibomian gland dysfunction. Its unique mechanism stabilizes the tear film’s lipid layer, reducing evaporation and possibly liquefying altered secretions. It is administered as one drop four times daily per eye, improving symptoms and showing good tolerability.
Dry Eye Disease Treatment Market Evolves with TRYPTYR’s Regulatory Milestone
Six years after separating from Novartis and becoming a publicly traded company, eye care specialist Alcon has received its first FDA approval for a prescription dye eye disease medication. The approval is for TRYPTYR (acoltremon ophthalmic solution), a treatment for dry eye disease.
TRYPTYR is a novel TRPM8 receptor agonist designed to stimulate tear production and is administered as a single drop twice daily. This approval is based on data from two Phase III dry eye disease clinical trials involving over 930 patients with a history of DED, randomized equally to receive either TRYPTYR or a placebo vehicle.
In the COMET-2 and COMET-3 studies, up to four times more patients treated with TRYPTYR showed at least a 10mm increase in natural tear production by Day 14 compared to those receiving the vehicle—42.6% versus 8.2% in COMET-2, and 53.2% versus 14.4% in COMET-3 (both with p-values less than 0.0001). These significant improvements were consistent through Day 90, with TRYPTYR showing a statistically meaningful increase in tear production as early as the first day of treatment.
The approval of TRYPTYR has intensified the competition among the other pharma companies to bring their lead assets to the market.
Promising Dry Eye Disease Therapies on the Horizon
The evolving dry eye disease drug pipeline encompasses a diverse range of medications with innovative mechanisms, user-friendly administration, and enhanced safety and effectiveness. Several pharma companies are working on their lead candidates in various phases of development to improve the dry eye disease treatment landscape.
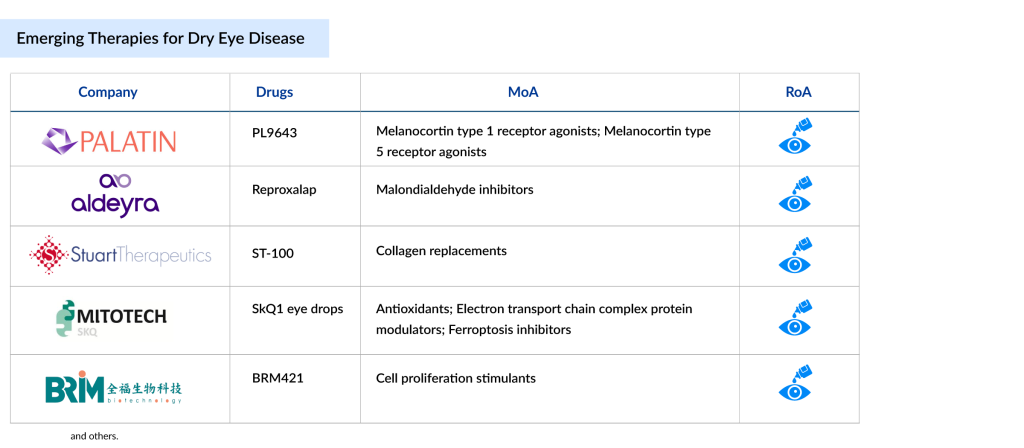
Some of the dry eye disease drugs in the pipeline include PL9643 (Palatin Technologies), Reproxalap (Aldeyra Therapeutics, Inc.), Tivanisiran (SYL1001) (Sylentis/PharmaMar), Tavilermide (Mimetogen), SkQ1 eye drops (Mitotech), BRM421 (BRIM Biotechnology), RGN-259 (Tβ4) (ReGenTree/RegeneRx Biopharmaceuticals), and others.
The anticipated launch of these emerging therapies for dry eye disease treatment are poised to transform the market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the dry eye disease treatment market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Where is the Dry Eye Disease Treatment Market Headed?
The landscape of dry eye disease treatment across the 7MM is rapidly evolving. A wide range of options, including pharmaceutical drugs, in-office procedures, eye protection strategies, and surgical solutions, are crucial in managing DED. Market forecasts indicate robust growth, with the DED market in the 7MM expected to exceed USD 3.7 billion by 2023.
The United States led the 7MM dry eye disease market in 2023, contributing approximately 73% of the total market share, and is projected to maintain its dominant position moving forward. This sustained growth is largely driven by the anticipated launch of several new therapies currently in development.
Additionally, the integration of diagnostic technologies for early detection and personalized treatment approaches is enhancing patient outcomes. Emerging markets are also contributing to demand growth as healthcare infrastructure improves globally.
Overall, the dry eye disease market is headed toward more effective, targeted therapies combined with technological advancements, creating a dynamic and rapidly evolving landscape.

Downloads
Article in PDF
Recent Articles
- FDA Expands SOLIRIS for Pediatric Myasthenia Gravis; vTv’s Cadisegliatin Program Resumes as FDA L...
- Exploring the Growing Demand and Evolving Market Dynamics of Artificial Tears
- Upcoming Contenders in Dry Eye Disease Drug Space: 5 Therapies to Watch Post-TRYPTYR Approval
- Alcon Gains CE Mark for Clareon Vivity IOL in Europe; MicroPort MedBot’s Toumai SP Robot Wins NMP...
- Leo Pharma’s Hand Eczema Clinical Trial Updates; GSK’s PD-1 inhibitor Jemperli Approval; Ro...