EMA Biosimilars Approvals
Jul 04, 2014
In the European Union (EU), a legal framework for approving biosimilars was established in 2003. This framework means that biosimilars can only be approved centrally via the European Medicines Agency (EMA) and not nationally.
On 27 June 2014, the European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) had recommended granting of marketing authorization for a biosimilar insulin glargine product (LY2963016).
Eli Lilly and its partner Boehringer Ingelheim has produced the biosimilar insulin glargine, which will be called as Abasria (LY2963016). The drug is a biosimilar of French drugmaker Sanofi’s diabetes drug Lantus (insulin glargine).
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Teva sells generics; Valeant trades; Roche’s Genentech; J&J’s next potential fro...
- Could Amgen’s Biosimilar Wezlana Pose a Challenge to Johnson & Johnson’s Stelara
- Sandoz’s Generic Revlimid; Agios’ Pyrukynd; Organon Announces 4Q & Full-year Earnings ...
- Pfizer uses; FDA approves; EMA approves; Mylan launches
- Biosimilars: A benchmark in Pharmaceutical Business
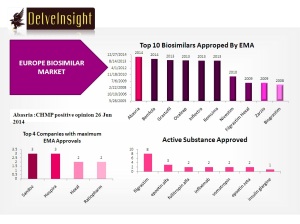
To date, EMA has approved 21 biosimilars within the product classes of human growth hormone, granulocyte colony-stimulating factor, erythropoietin, insulin and TNF-inhibitor, for use in the EU.
Two biosimilar approvals have been withdrawn; one for filgrastim in April 2011 and one for somatropin in May 2012, leaving a total of 19 biosimilars approved for use in Europe.
Share This post on twitter #Migraine is a medical condition characterized by recurrent headaches that can last from 4 to @abbvie 72 hours and often results in a wide range of symptoms. It is mainly associated with a painful headache
Downloads
Article in PDF
Recent Articles
- EMA to relocate to Amsterdam; Roche’s prospects; Amgen’s Humira Biosimilar delayed; Purdue’s opio...
- Biosimilars: A benchmark in Pharmaceutical Business
- Analyzing the Key Trends Driving the Biosimilar Market Growth
- Sandoz’s Generic Revlimid; Agios’ Pyrukynd; Organon Announces 4Q & Full-year Earnings ...
- Progressive Supranuclear Palsy– A neurodegenerative disorder