New Approved PML Treatment Options – A Long-awaited Hope for PML Patients
Aug 05, 2022
Progressive multifocal leukoencephalopathy is a severe, progressive, and lethal demyelinating disease that presents in immunocompromised states. It is a rare indication, with just over 1.5K PML diagnosed incident cases in the United States, per DelveInsight analysis. It occurs in the setting of predisposing immunosuppressive conditions like HIV, hematological malignancies, and treatment with chemotherapy and autoimmune medications. According to our estimates, in the United States, >900 patients develop PML due to HIV infection, followed by hematological malignancies (around 300 cases) and chemotherapy medications (>200 cases). Autoimmune medications result in ~150 cases of PML.
However, in the last few decades, cases of PML have not increased significantly, primarily due to the availability of effective PML treatment options like antiretroviral therapy (ART), which has enhanced outcomes for HIV patients leading to fewer HIV-infected PML cases. Also, stringent clinical regulations and monitoring by FDA and EMA have limited the use of natalizumab, efalizumab, and rituximab, resulting in a reduced incidence of drug-associated PML. In France, a substantial decline in natalizumab-associated PML incidence has been reported as a result of persistence and reinforcement of educational activities and risk-minimization tactics in managing disease-modifying therapies for multiple sclerosis. Similarly, the Swedish nationwide PML Risk Surveillance Program seemed to have lowered the PML incidence in Sweden. However, with increased approvals of biologics in different indications, the chances of developing PML remain substantial.
Nevertheless, PML is a fatal condition, and as per published literature, in several cases, survival rates for PML patients range from 40% to 50% in the first few months after diagnosis. Currently, there is no established PML treatment.
Downloads
Click Here To Get the Article in PDF
Recent Articles
The existing PML treatment strategy aims at restoring immune function to improve survival successfully. Antiretroviral therapy and removing or decreasing any potential source of immunosuppression are the recommended PML treatment options. Agents such as cytosine arabinoside, cidofovir, risperidone, mirtazapine, and mefloquine are majorly used to manage PML.
Cytarabine (cytosine arabinoside) was the first drug thought to be beneficial against infection via DNA polymerase inhibition. Despite being reported unsuccessful in numerous circumstances. Mefloquine is an antimalarial drug with significant CNS penetration and action against JCV. In vitro investigations have revealed that mefloquine may be effective in some situations.
Moreover, Immune modulation therapy aims to restore protective anti-JCV responses in PML or to suppress excessive immune responses in PML-IRIS. The first category includes cytokines and antiretroviral drugs used to treat HIV+ patients. Type 1 interferons (IFNs) have been produced to treat or prevent viral diseases such as hepatitis B and C virus and oral human papillomavirus. The second class of immune modulators consists of medicines that suppress an overly aggressive immune response to JCV-infected brain tissue.
Although corticosteroids are now used as a standard of care for PML-IRIS, no controlled trials have been undertaken to demonstrate their efficacy. Adoptive T-cell therapy, checkpoint inhibitor therapy, interferon-alpha-2A, interleukin-2 inhibitor, IV immunoglobulins, and plasmapheresis are some of the other therapies used for PML treatment.
However, the prognosis for the huge number of PML patients remains poor. Also, as stated, immune reconstitution is desired for the management of PML and results in better outcomes; on the contrary, PML treatment may also result in Immune Reconstitution Inflammatory Syndrome (IRIS), a potentially precarious amount of acute and often fulminant inflammation in the brain that ought to be treated.
How Can Pharmaceutical Companies Cater to the Needs of PML Patients?
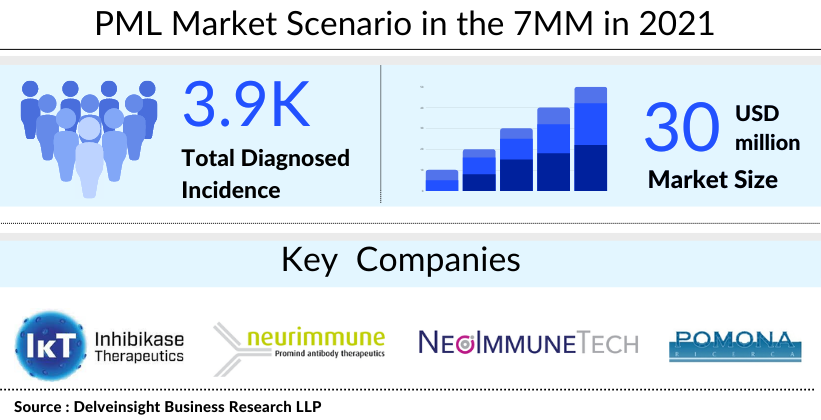
DelveInsight estimates show that the United States accounted for the highest PML market size as compared to Japan and EU5. The total PML market size was found to be close to USD 30 million in 2021, which is expected to increase by 2032.
Currently, the PML pipeline mainly comprises early Phase I or preclinical assets owing to a lack of robust development. Only a few companies are investigating potential PML treatment and management options. For instance, NeoImmuneTech is developing NT-I7 (efineptakin alfa), a human IL-7 fusion protein designed to overcome the key limitations of endogenous IL-7 in infectious diseases and other indications. The company is discovering the utility of IL-7-based therapeutics in enhancing immune function. Additionally, Inhibikase Therapeutics is investigating kT-01427 to block the causative virus of PML from replicating in the body. IkT-01427 is an oral, brain-penetrant medication for systemic administration intended to block the entry of the JC virus into the cell, thereby inhibiting the virus’ ability to replicate in the body. It is a new molecular entity developed with the company’s RAMP™ technology and is currently in the preclinical development phase.
Another player, Neurimmune, is exploring NI307, a monoclonal antibody targeting JC polyomavirus or the PML treatment. Presently, it is in the discovery phase. Moreover, another company, Pomonaricerca, is evaluating PN-JC 1 for PML treatment. PN-JC 1 is a fully human monoclonal antibody. It is an IgG1 molecule with potent antiviral neutralization action. The drug, which is currently in the early stages of development for PML treatment, could be effective in the prevention of natalizumab-induced PML as well as the treatment of the ouvert disease.
Although PML is rare, with growing accessibility and use of mAbs in different indications, the risk of developing PML remains substantial. Besides, the dearth of clinical development and lack of effective PML treatment warrants the need for novel disease-modifying/curative options; thus, PML leaves enormous opportunities for pharmaceutical players to investigate this therapy area and cater to the needs of patients.

FAQs
Progressive multifocal leukoencephalopathy is a severe, progressive, and lethal demyelinating disease that presents in immunocompromised states. It is a rare indication, with just over 1,500 diagnosed incident cases in the United States, per DelveInsight analysis.
The early PML symptoms might vary from person to person, depending on which nerves are affected first. The common PML symptoms include difficulty in walking, loss of vision, personality changes, muscle weakness, and others.
MRI is the most common method used for PML diagnosis. If MRI doesn’t gave a clear picture, then biopsy is recommended.
The existing PML treatment strategy aims at restoring immune function to improve survival successfully. Antiretroviral therapy and removing or decreasing any potential source of immunosuppression are the recommended PML treatment options. Agents such as cytosine arabinoside, cidofovir, risperidone, mirtazapine, and mefloquine are majorly used to manage PML.
Only a few companies, such as NeoImmuneTech, Inhibikase Therapeutics, Neurimmune, and Pomonaricerca are investigating potential PML treatment and management options.
The promising PML therapies in the pipeline include NT-I7 (efineptakin alfa), kT-01427, NI307, and PN-JC 1
Downloads
Article in PDF