Role of TLR7/8 Agonists in Treating Various Cancers
Apr 10, 2023
Table of Contents
The Toll family of receptors includes several related proteins involved in plant and animal development and defense. Mammalian TLRs are a large family with at least 11 members, of which humans encode the first ten. Individual TLRs are critical in recognizing pathogen-derived microbial components such as bacteria, fungi, protozoa, and viruses. The toll was discovered at the end of the twentieth century in Drosophila melanogaster as a gene controlling the developing embryo’s dorsal-ventral axis. One year later, it was discovered that a mammalian homolog of the Toll receptor (now known as TLR4) could induce the expression of genes involved in inflammatory responses. Following the identification and naming of the first mammalian TLR, several proteins that are structurally similar to TLR4 were identified and named Toll-like receptors.
Individual TLRs are distributed differently within the cell. TLR1, TLR2, and TLR4 are found on the cell surface, whereas TLR3, TLR7, TLR8, and TLR9 have been found in intracellular compartments such as endosomes. The cytoplasmic portion of TLRs is very similar to the IL-1 receptor family and is referred to as a Toll/IL-1 receptor (TIR) domain. Other distinguishing features of TLR receptor structure include the presence of leucine-rich repeats (LRRs) in the extracellular domain and short cysteine-rich patches.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- 4DMT Presents Data From Phase II PRISM Clinical Trial; Adaptimmune Announces FDA Acceptance of Bi...
- Smith+Nephew’s Hip Arthroplasty System; Mammotome Launched the HydroMARK Plus Breast Biopsy Site ...
- Vaccine Adjuvants: Enhancing Immunity in the Fight Against Diseases
- FDA Approves Opdivo Qvantig™ for Solid Tumors; Nuvation Bio’s Taletrectinib NDA Accepted for ROS1...
- Lilly to Acquire Versanis; FDA Approves Beyfortus for RSV in Infants; Alnylam Presented Updates o...
Toll-like receptors (TLR) 7 and 8 are functionally localized to endosomes and recognize specific RNA sequences. They play critical roles in initiating innate and adaptive immune responses. TLR7/8 activation both protects the host from invading pathogens and boosts immune responses. In contrast, persistent TLR7/8 signaling causes immune overreaction. As a result, TLR7/8 agonists and antagonists are promising drug candidates for the treatment of immune disorders.
Signaling of TLR7/8
TLR7 and TLR8 mediate the recognition of purine-rich ssRNA to elicit immune responses against pathogens recognized in the endosome. Among the naturally derived viral uridine-rich ssRNAs are influenza and HIV. TLR7 and TLR8 are also involved in bacterial RNA recognition. TLR7 and TLR8 both signal via MyD88/MAL, including IRAK-4 recruitment, to mediate cytokine and IFN production via NF-κB and IFN regulatory factor (IRF) 7, respectively. TLR7 and TLR8 transcriptions are activated in response to proinflammatory cytokine signaling via NF-κB. TLR7 and 8 are released from the endoplasmic reticulum (ER) to the endosome by leucine-rich repeat (LRR)-containing protein 59 (LRRC59), which promotes the ER-resident membrane protein uncoordinated 93 homolog B1 (Caenorhabditis elegans) (UNC93B1) to mediate TLR7 vesicle packaging and translocation to the Golgi.
Role of TLR7/8 Agonist in Immune Disorders and Cancers
Several studies have linked TLR to the development of immune disorders. In many diseases, constitutive activation of innate immune cells is caused by defective IL-10 signaling. TLR-mediated microbial recognition in the intestine, for example, triggers the development and progression of chronic enterocolitis. The TLR-MyD88-dependent pathway appears to be involved in allograft rejection, atherosclerosis, and rheumatoid arthritis development. TLR recognition of commensal bacteria has also been shown to be important in maintaining intestinal epithelial homeostasis. Thus, even without infection, TLR-mediated pathways are likely involved in many aspects of immune responses.
Many TLR antagonists are in clinical trials for such conditions. A toll-like receptor 7 and 8 (TLR7/8) agonist with immunostimulant and antitumor properties. TLR7/8 agonist MEDI9197 binds to and activates TLR7 and 8, stimulating antigen-presenting cells (APCs), including dendritic cells, after intratumoral administration (DCs). When DCs are activated, they produce proinflammatory cytokines and activate cytotoxic T-lymphocyte (CTL) and B-lymphocyte immune responses. This may result in tumor cell lysis. TLR7 and TLR8, members of the TLR family, play critical roles in immune system activation.
TLR7-mediated type I IFNs, a family of key antiviral cytokines that induce a variety of genes collectively known as IFN-inducible genes, are produced in response to ssRNA viruses. Viruses have developed evasive strategies to avoid and shut down type I IFN responses. There are several innate viral sensors responsible for inducing type I IFN (these include melanoma differentiation-associated protein 5), laboratory of genetics and physiology 2 (LGP2), mitochondrial antiviral-signaling protein (MAVS), stimulator of IFN genes (STING), and retinoic acid inducible gene (RIG)-I).
Promising TLR7/8 Agonist in Research and Development
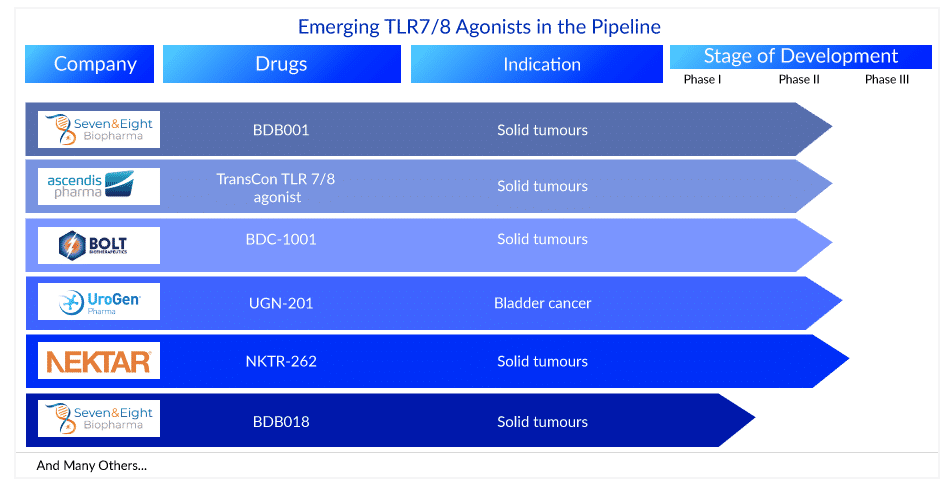
There are currently no TLR8 selective agonists approved for clinical use. Over 10+ leading pharma companies are currently working on developing novel TLR7/8 agonists. TransCon TLR 7/8 agonist (Ascendis Pharma), BDC-1001 (Bolt Biotherapeutics), and BDB001 (Seven and Eight Biopharmaceuticals) are TLR7/8 agonists in clinical trials for various cancers. Many TLR7/8 agonist clinical trials attempt to boost anti-tumor activity by using agonists that target both TLR7 and TLR8 targets. More TLR7/8 agonists clinical trials targeting TLR8 selectively, on the other hand, are gaining attention.
Some of the TLR7/8 agonists in the mid and late stages of development are –
BDB001: Seven and Eight Biopharmaceuticals
BDB001 (Seven and Eight Biopharmaceuticals) is an immune modulator that activates dendritic cells, triggering both innate and adaptive immune responses against cancer. BDB001 is a first-in-class TLR7/8 agonist that is administered intravenously, allowing for a more comprehensive treatment of solid tumors. The drug is currently in Phase II testing for the treatment of solid tumors. Previously, Seven and Eight Biopharma reported that intravenous administration of BDB001, both as monotherapy and in combination with an anti-PD-1 mAb, results in favorable tolerability and robust systemic immune activation, leading to durable clinical responses in a variety of advanced solid tumor types. The presentation at SITC 2021 provides interim safety and efficacy results from a Phase I dose escalation/expansion trial of BDB001 in combination with atezolizumab in advanced solid tumors. The findings show that BDB001, in combination with atezolizumab, is well tolerated, with evidence of robust immune activation leading to clinical responses in multiple tumor types.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TransCon TLR 7/8 agonist: Ascendis Pharma
TransCon TLR7/8 Agonist developed by Ascendis Pharma is an investigational long-acting prodrug of resiquimod, a small molecule agonist of Toll-like receptors (TLR) 7 and 8, designed to provide sustained activation of intratumoral antigen-presenting cells driving tumor antigen presentation and induction of immune stimulatory cytokines with a single intratumoral injection for weeks or months. Ascendis Pharma is currently conducting the transcendIT-101 Study, a Phase I/II study to evaluate TransCon TLR7/8 Agonist as monotherapy or in combination with pembrolizumab in dose escalation and dose expansion cohorts.
Ascendis Pharma A/S announced new data from the dose-escalation portion of transcendIT-101, the company’s Phase I/II open-label, multi-center trial of TransCon TLR7/8 Agonist in patients with advanced solid tumors, in November 2022. The dose escalation data from transcendIT-101, presented by Diwakar Davar, M.D. of the University of Pittsburgh Medical Center during the annual meeting of the Society for Immunotherapy of Cancer (SITC 2022), suggests that intratumoral TransCon TLR7/8 Agonist was safe and well-tolerated alone or in combination with pembrolizumab; that it demonstrated target immune system engagement in injected and non-injected tumors along with a systemic immune response; and that it showed early signs of clinical activity alone or in combination with pembrolizumab.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All 23 patients enrolled in the trial’s dose-escalation phase had advanced or metastatic solid tumors that had progressed on prior treatments, with 9 in the monotherapy cohort (intratumoral TransCon TLR7/8 Agonist alone) and 14 in the combination therapy cohort (intratumoral TransCon TLR7/8 Agonist plus the check-point inhibitor pembrolizumab). Two dose levels were tested: 0.3 mg/lesion and 0.5 mg/lesion. The Phase 2 dose was declared to be 0.5mg/lesion for up to two lesions.
BDC-1001: Bolt Biotherapeutics
BDC-1001 is an immunostimulating antibody conjugate (ISAC) that is being developed for the treatment of patients with HER2-expressing cancer. Recently, Bolt Biotherapeutics, Inc. reported positive topline data from its recently completed dose-escalation study of BDC-1001 in HER2-expressing solid tumors, indicating that the company will proceed to two Phase II studies. The trial’s topline results show that BDC-1001 was well tolerated at all dose levels and schedules tested, both as monotherapy and in combination with nivolumab. More frequent administration, including every other week (q2w) and weekly (q1w), achieved target drug exposure levels at or near the recommended Phase II dose (RP2D). Multiple partial responses (PRs), tumor shrinkage, and long-term stable disease at or near the RP2D were observed in multiple HER2-expressing solid tumor types in monotherapy and in combination with nivolumab. Furthermore, biomarker data show that clinical and safety data are related to the ISAC mechanism. These findings support the selection of an RP2D and the progression to Phase II studies. More than 100 patients with 16 different HER2-expressing solid tumor types were enrolled in Bolt Biotherapeutics’ Phase I dose-escalation trial. At the time of enrollment, all patients had evidence of tumor progression following prior standard of care treatments, and the majority of them had been heavily pre-treated.
Apart from these, several TLR7/8 agonists such as UGN-201 (UroGen Pharma), NKTR-262 (Nektar Therapeutics), BDB018 (Seven and Eight Biopharmaceuticals), and others are also in the pipeline for various cancers.
What’s Cooking in the TLR7/8 Agonist Space?
- In March 2023, Bolt Biotherapeutics, Inc., a clinical-stage biopharmaceutical company pioneering novel immuno-oncology therapeutics for the treatment of cancer, announced positive topline data from the company’s recently completed dose-escalation study of BDC-1001 in HER2-expressing solid tumors, supporting the company’s progression into two Phase II studies.
- In November 2022, Ascendis Pharma A/S announced new findings from the dose-escalation phase of transcendIT-101, the company’s Phase I/II open-label, multi-center trial of TransCon TLR7/8 Agonist in patients with advanced solid tumors.
- In February 2022, Primmune Therapeutics, presented interim data related to PRTX007, a novel, orally administered, small molecule toll-like receptor 7 (TLR7) specific agonist that is currently in Phase I development, at the Conference on Retroviruses and Opportunistic Infections (CROI). Oral administration of PRTX007 in this first-in-human study of healthy volunteers exhibited a favorable safety profile, rapid absorption and conversion to TLR7 agonist PRX034, and activation of the innate immune system, without causing inflammation.
- In November 2021, Seven and Eight Biopharmaceuticals Inc., a clinical stage biotechnology company developing proprietary novel immuno-oncology therapies to activate the immune system against cancer, announced that interim Phase I data for BDB001 in combination with atezolizumab in advanced solid tumours will be presented at the 2021 Society for Immunotherapy of Cancer (SITC) Annual Meeting.
What Lied Ahead in the TLR7/8 Agonists Domain?
Preclinical and clinical evidence suggests that synthetic TLR7/8 ligands have the potential to be powerful immunomodulators, vaccine adjuvants, and cancer therapeutics, but challenges in achieving appropriate efficacy while avoiding toxicities remain a significant barrier. Recent crystal structure elucidation, SAR analyses, and delivery approaches have resulted in steady progress, though systemic administration of TLR7/8 agonists remains difficult. Furthermore, defining optimal dosing thresholds and timing intervals will be critical, given that research on these compounds has closely followed progress on synthetic TLR4 and TLR9 agonists, where significant efforts have been made towards understanding tolerability and circadian effects.
Although TLR7 tolerance has been observed in numerous studies, the mechanism of induction is still under investigation. Initial research suggests that dosing schemes that avoid TLR7 tolerance can be developed, though this phenomenon needs to be studied further to ensure maximum efficacy of these compounds. Furthermore, the dependence of TLR expression and agonist activity on circadian biology has yielded promising results in other agonist systems but has yet to be thoroughly investigated for TLR7/8 agonism. Finally, as briefly mentioned in the previous sub-section, defining the synergistic role of synthetic TLR7/8 agonists in combination with established as well as emerging treatments is critical in our pursuit of long-term cures for cancer, infectious diseases, and allergic diseases.

Downloads
Article in PDF
Recent Articles
- Eli Lilly’s COVID-19 Antibody trial; Approval for Oriahnn; Gilead’s Remdesivir Clinic...
- Toll-Like Receptor Modulators: Emerging therapy for many indications
- Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR...
- FDA Approves Opdivo Qvantig™ for Solid Tumors; Nuvation Bio’s Taletrectinib NDA Accepted for ROS1...
- Lilly to Acquire Versanis; FDA Approves Beyfortus for RSV in Infants; Alnylam Presented Updates o...