Huge Unmet Needs in the Glioblastoma Multiforme Treatment Market Driving the Market Size Growth
Oct 10, 2024
Headaches, blurred vision, nausea, lack of appetite, and mood swings. Sound familiar? These are common complaints that most of us shrug off as stress or fatigue. However, these seemingly minor issues are also early glioblastoma symptoms, warning signs of one of the most aggressive and deadly forms of brain cancer—glioblastoma multiforme (GBM). Often masked by their vagueness, these early signs make it difficult to detect the disease until it’s in an advanced stage. As a highly malignant tumor, GBM is notorious for its rapid progression and spread. Understanding what is glioblastoma and recognizing its subtle onset is critical for timely intervention. The stakes are high, with the glioblastoma survival rate remaining low despite advances in glioblastoma treatment. Once symptoms worsen, such as severe headaches or cognitive decline, it signals that the disease is advancing. Knowing what are the signs that glioblastoma is getting worse could be a matter of life and death.
GBMs are rare tumors with a global incidence of less than 10 per 100,000 people.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Merck’s Gefapixant; Pfizer’s Somatrogon; Gilead’s Viklury; AbbVie’s Skyrizi; Gilead’s...
- Glioma vs. Glioblastoma Therapeutics Space: Unveiling the Battlefront
- Idera’s Phase I Data; DelMar Initiates Trial; Novartis’ study; Humira gets EC approval
- 13 of the most commonly asked questions about Glioblastoma multiforme, Answered
- Rare Cancer Market: A Global Crusade on what is a ‘less common cancer’?
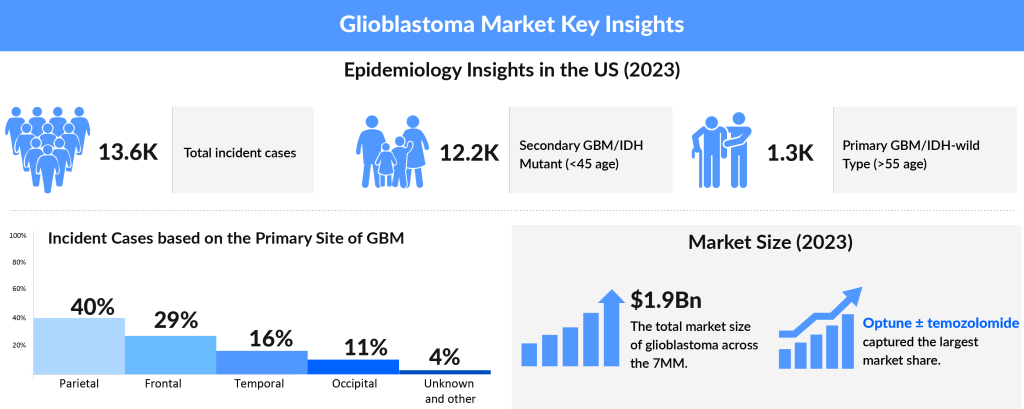
In 2023, the US recorded the highest number of incident glioblastoma cases among the 7MM, with approximately 13,600 cases. Within the EU4 and the UK, Germany had the most incident cases, followed by France. Specifically in the US, there were about 12,200 cases of Primary GBM/IDH-wild Type and 1,360 cases of Secondary GBM/IDH Mutant. DelveInsight estimates that the age group of 65–74 years accounted for the highest number of cases, with those aged 55–64 years following closely behind.
DelveInsight
Furthermore, Glioblastoma multiforme appeared to have a higher male predisposition. In a symposium presented at the Society of NeuroOncology in 2018, gender discrepancy at all the levels – cellular, systemic, and genetic, in Glioblastoma multiforme was largely pressed, with over 60% of the diagnosed population constituting males. It could be either due to sex hormones or due to IDH-1 mutation, the frequency of which is quite higher in males.
Glioblastoma multiforme epidemiology analysis further revealed that the majority of the GBM cases, more than 90%, were reported to have Primary glioblastoma. GBMs either develop de novo, known as or they may progress from low-grade or anaplastic astrocytomas, termed as Secondary glioblastoma. Although the tumors share similar histologic appearance, they differ largely in terms of precursor cells, thus, calls for different therapeutic approaches.
Even though GBM tumors are rare, the median survival rate of Glioblastoma multiforme is 10-15 months after diagnosis – attributable to insufficient knowledge of the early stages of Glioblastomas. Delayed diagnosis is one of the significant reasons behind poor patient outcomes and a major unmet need in the market, besides, of course, treatment. To date, Glioblastomas remain incurable and available treatment is palliative in nature. The cardinal aim of the treatment of patients newly diagnosed with GBM is to achieve “maximum safe resection” by performing surgery. Although the metastatic property of Glioblastoma multiforme is a rare scenario, i.e., they never leave the nervous tissue, however, the tentacles and finger-like projections of the brain cells infiltrate the brain holistically, making it difficult to remove altogether. In the cases where surgery seems to be out of the question or fails in achieving optimal results, physicians turn to Radiotherapy and chemotherapy as concomitant or adjuvant therapy. At present, the Glioblastoma multiforme treatment market is inhabited with a few FDA approved chemotherapy drugs namely BiCNU (carmustine), Avastin (bevacizumab), and Temodar (temozolomide). Schering-Plough’s Temodar is the most frequently used chemo drug. Temodar is the most widely used chemotherapy and manages to increase the median survival rate by a few weeks, and so does the other two.
Regardless of advanced technology, diagnostic modalities, R&D in brain neoplasms, carefully weighted multidisciplinary treatment approach, the overall five-year survival rate is still around 5% from the past decades, which is one of the huge unmet needs in the GBM market. Average survival does not traverse the one-year mark; and if the tumor recurs, this survival rate pales further, with almost all the GBM patients’ tumor progressing and ending with only the death of the patient.
The treatment of brain tumors faces unique challenges; the presence of the blood-brain-barrier (BBB) is one of the most notable ones. Unfortunately, therapeutic options available in the current Glioblastoma multiforme find themselves incapable of crossing it. It is highly argued that the GBM is responsible for a leaky BBB; however, it does not help as the cancerous cell remains within the precincts of compact BBB. Another hurdle that perverses in the Glioblastoma multiforme treatment market is Intertumor heterogeneity, and this is why in the very first place, the tumor is termed as Glioblastoma ‘multiforme’. Intertumor heterogeneity is a root cause of treatment resistance and failure resulting in mean survival less than 14 months. Further, there is no therapeutic option or standard care available to approach recurring GBM cases.
Unmet Needs in GBM Treatment Market
To address Unmet needs in the Glioblastoma multiforme treatment market, there is a dire need of funding. Being a rare disease, there is already a dearth of patient accrual, and understanding of tumor and the current scenario does not look promising even in terms of funds needed for the development of novel therapeutic targets. Innumerous clinical trials are underway that are majorly led by Industries, followed by academia with a focus on systemic therapies. There is no remarkable progress in patient outcome, rather the results appear to be quite disappointing. Not to forget, clinical trial enrollment for such a rare brain tumor is not less than an uphill struggle. The studies underway, and in the pipeline, always face the issues with selection bias, confounders, and inappropriate historic controls.
Several pharmaceutical companies, including Bayer, Chimerix, Aivita Biomedical, Denovo Biopharma, Northwest Therapeutics, VBL Therapeutics, Laminar Pharmaceuticals, MedImmune, DNAtrix, Immunomic Therapeutics, Imvax, MimiVax, CNS Pharmaceuticals, Epitopoietic Research Corporation (ERC), Istari Oncology, SonALAsense, and many others, are running clinical trials investigating their candidates in different stages of clinical development, but most of them are in the Phase II stage of development. However, with a bleak rate of only a few reaching Phase III clinical development, it is yet to see which candidate stands the test of time.
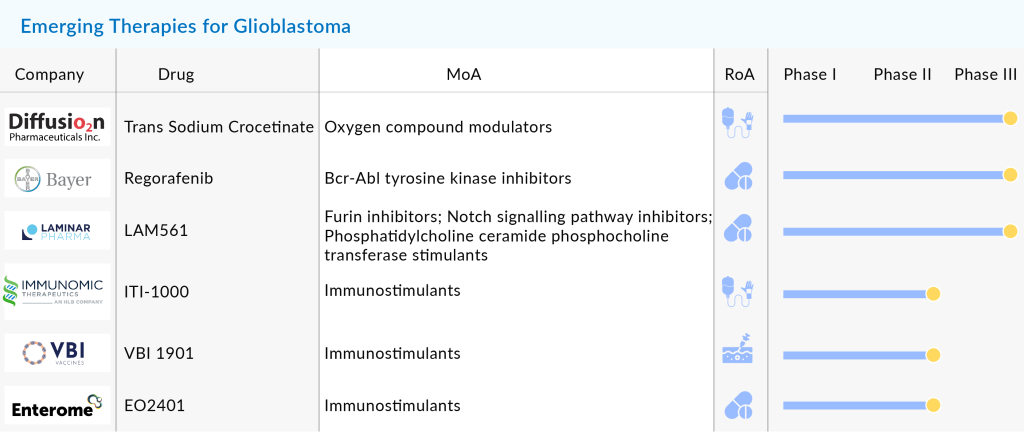
However, there is a drug that is set for approval soon. LAM561 (2-hydroxyoleic acid) is an oral synthetic derivative of oleic acid that can cross the Blood-Brain Barrier to target brain cells. It alters the plasma membrane composition in cancer cells, reducing the activity of signaling proteins that promote tumor growth. Promising results have been seen in treating aggressive glioblastomas, and the drug is currently in a Phase II/III trial. According to the company’s pipeline, LAM561 is expected to receive approval for adult glioma in 2024 and for pediatric glioma by 2025/2026.
Advances have been made in neuroimaging, in the field of surgical techniques, however, none seems to have addressed the survival crisis. The grim prognosis, miserable patient outcomes, and temporizing treatment approaches scream for distinct options in the GBM treatment market landscape to budge the five-year survival rate. With only a handful of the approved therapies available in the present GBM treatment market, it is imperative to understand the challenges that plague the emergence of new therapeutic targets. Comprehensive knowledge of the molecular pathology or etiology of the disease further offers new handles in moving the needle in the Glioblastoma multiforme treatment market and meeting the unmet needs.

Downloads
Article in PDF
Recent Articles
- Glioma vs. Glioblastoma Therapeutics Space: Unveiling the Battlefront
- Glioblastoma Multiforme Market: Emerging Pipeline Therapies To Keep A Keen Eye On
- Idera’s Phase I Data; DelMar Initiates Trial; Novartis’ study; Humira gets EC approval
- Glioblastoma Multiforme: Advancements in the Treatment Paradigm of the Malignant Condition
- Merck’s Gefapixant; Pfizer’s Somatrogon; Gilead’s Viklury; AbbVie’s Skyrizi; Gilead’s...